UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
______________________________________
FORM 10-K
______________________________________
(Mark One)
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the fiscal year ended December 31 , 2024
OR
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |||||
For the transition period from ______ to ______
Commission file number 333-279382
______________________________________
QT Imaging Holdings, Inc.
______________________________________
(Exact Name of Registrant as Specified in its Charter)
| (State or Other Jurisdiction of Incorporation or Organization) | (I.R.S. Employer Identification No.) | |||||||
Chief Executive Officer
Telephone: (650 ) 276-7040
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||||||
Securities registered pursuant to section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes o No x
_________________
1QT Imaging Holdings, Inc. (the “Company”) has received written notice from The Nasdaq Stock Market LLC (“Nasdaq”) that it has commenced proceedings to delist the Company’s common (ticker symbol: QTI) from Nasdaq, and suspended trading in the Company’s common stock pending the completion of such proceedings. As a result, effective January 28, 2025, the Company’s common stock commenced trading in the over‑the-counter market under the symbol “QTIH”, and the trading of the common stock was upgraded to the OTCQB Venture Market on March 11, 2025.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,”and "emerging growth company" in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | o | Accelerated filer | o | |||||||||||
| x | Smaller reporting company | |||||||||||||
| Emerging growth company | ||||||||||||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act.
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements.
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b).
o
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act).
Yes o No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant, based on the closing price of the registrant’s common stock on The Nasdaq Stock Market LLC as of the last business day of the registrant’s most recently completed second fiscal quarter, June 30, 2024, was $9.2 million. Shares of common stock beneficially owned by each executive officer, director, and holder of more than 10% of the registrant’s common stock have been excluded in that such persons or entities may be deemed to be affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date. 27,653,210 shares of common stock were outstanding at March 28, 2025.
Table of Contents
| Page | |||||
| Cautionary Statement About Forward-Looking Statements | ii | ||||
| Summary of Risk Factors | iv | ||||
| 96 | |||||
i
CAUTIONARY STATEMENT ABOUT FORWARD-LOOKING STATEMENTS
This annual report contains forward-looking statements. All statements, other than statements of present or historical fact included in or incorporated by reference in this annual report, regarding the future financial performance of QT Imaging Holdings, Inc. (the “Company”), as well as the Company’s strategy, future operations, financial position, estimated revenues, and losses, projected costs, prospects, plans and objectives of management are forward-looking statements. When used in this annual report, the words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “should,” “will,” “would” the negative of such terms and other similar expressions are intended to identify forward-looking statements, although not all forward‑looking statements contain such identifying words. These forward-looking statements are based on management’s current expectations, assumptions, hopes, beliefs, intentions and strategies regarding future events and are based on currently available information as to the outcome and timing of future events. The Company cautions you that these forward-looking statements are subject to all of the risks and uncertainties, most of which are difficult to predict and many of which are beyond the control of the Company, incident to its business.
These forward-looking statements are based on information available as of the date of this annual report, and current expectations, forecasts and assumptions, and involve a number of risks and uncertainties. As a result of a number of known and unknown risks and uncertainties, the Company’s actual results or performance may be materially different from those expressed or implied by these forward-looking statements. Some factors that could cause actual results to differ include:
•the Company’s ability to recognize the anticipated benefits of the Business Combination (as defined below), which may be affected by, among other things, competition and the ability of the Company to grow and manage growth profitably following the closing of the Business Combination;
•changes in applicable laws or regulations;
•the outcome of any legal proceedings against the Company;
•the financial and business performance of the Company, including financial projections and business metrics and any underlying assumptions thereunder;
•the Company’s ability to successfully and timely develop, sell and expand its technology and products, and otherwise implement its growth strategy;
•risks relating to the Company’s operations and business, including information technology and cybersecurity risks, loss of customers and deterioration in relationships between the Company and its employees;
•risks related to increased competition;
•risks that the Company experiences difficulties managing its growth and expanding operations;
•the impact of geopolitical, macroeconomic and market conditions;
•the ability to successfully select, execute or integrate future acquisitions into the business; and
•other risks and uncertainties set forth in this annual report in the section entitled “Risk Factors” beginning on page 49.
Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur, and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. Some of these risks and uncertainties may in the future be amplified by events we do not expect or cannot predict. Additionally, new risk factors and uncertainties may emerge from time to time, and it is not possible for management to predict all risk factors and uncertainties. As a result of these factors, the forward‑looking statements in this annual report may not prove to be accurate.
Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained in this annual report, whether as a result of any new information, future events, changed circumstances or
ii
otherwise. You should read this annual report completely, and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
iii
SUMMARY OF RISK FACTORS
The below summary of risk factors provides an overview of many of the risks we are exposed to in the normal course of our business activities. As a result, the below summary risks do not contain all of the information that may be important to you, and you should read the summary risks together with the more detailed and complete discussion of risks set forth under the heading “Risk Factors” in Part I, Item 1A of this annual report, as well as elsewhere in this annual report. Additional risks, beyond those summarized below or discussed elsewhere in this annual report, may apply to our activities or operations as currently conducted or as we may conduct them in the future or in the markets in which we operate or may in the future operate.
Consistent with the foregoing, we are exposed to a variety of risks, including risks associated with the following:
Risks Associated with Our Business, Financial Condition and Need for Additional Capital
•We have incurred significant operating losses in the past and may never achieve or maintain profitability.
•We have a limited operating history with our current offerings, which makes it difficult to evaluate our current and future business prospects and increases the risk of your investment.
•If we are unable to attract new customers on a cost-effective basis, our business will be harmed.
•We may be unable to successfully execute on our growth initiatives, business strategies, or operating plans.
•The forecasts and projections herein are based upon certain assumptions, analyses, and estimates. If these assumptions, analyses, or estimates prove to be incorrect or inaccurate, our actual results may differ materially from those forecasted or projected.
•If we fail to attract and retain qualified personnel, our business could be harmed.
•Our management team has a limited history working together operating the Company and, as a result, our past results may not be indicative of future operating performance.
•Our ability to introduce new products and features is dependent on adequate development resources. If we do not adequately fund our development efforts, we may not be able to compete effectively and our business and operating results may be harmed.
•We rely on internet infrastructure, bandwidth providers, data center providers, other third parties, and our own systems for providing solutions to our customers, and any failure or interruption in the services provided by these third parties or our own systems could expose us to litigation and negatively impact our relationships with customers, adversely affecting our brand and our business.
•We may not successfully develop or introduce new and enhanced products that achieve market acceptance, or successfully integrate acquired products or services with our existing products, and our business could be harmed and our revenue could suffer as a result.
•If we successfully commercially launch the QT Scanner 2000 Model A, (the “QT Breast Scanner”), products under development that are cleared by the U.S. Food and Drug Administration (the “FDA”) and other regulatory agencies, and they do not achieve widespread market acceptance, we will not be able to generate the revenue necessary to support our business.
•Recent changes in the United States related to payment policies for imaging procedures could have a negative impact on the utilization of our imaging services.
•In order to support the growth of our business, we may need to incur additional indebtedness under our credit facilities or seek capital through new equity or debt financings, which sources of additional capital may not be available to us on acceptable terms or at all.
Risks Associated with Healthcare Industry Shifts and Government Regulation
•Our business is subject to extensive pre-market and post-market government regulations and if we fail to comply with present or future regulator requirements that are applicable, it may subject our company to enforcement
iv
action by the FDA which may have a material adverse effect our business, financial condition and results of operations.
•Our products must be manufactured in accordance with federal, state and foreign regulations as to the methods used in, and the facilities used for, the manufacture of our products and if we, or our third-party manufacturers do not take the necessary steps to comply with applicable regulations, it could result in delays in the delivery of our products which could significantly and negative affect the supply of our products.
.Risks Associated with our Intellectual Property
•Our business relies upon a combination of patents and trade secrets to protect the intellection property related to our proprietary technologies and if we are unable to obtain and maintain intellectual property protections it could have an adverse impact on our market competitiveness and business prospects.
•Patents have a limited life span and given the amount of time required for the development, testing and regulatory review of products, patents protecting our future products might expire before or shortly after we commercialize these products which could result in us not having sufficient rights to exclude others from commercializing products similar or identical to ours for a sufficient amount of time, and as a result, we may not be able to obtain adequate protection of our patent portfolio.
Risks Associated with Our Management
•We are highly dependent on key members of our executive management team and the loss of members of our management team could result in a delay in the implementation of our business plan and plan of operations, which could result in a material adverse effect on our business, result of operations and financial condition.
•Most of the members of our management team have limited or no experience managing a publicly traded company and may not successfully or efficiently manage our transition to being a public company subject to regulatory oversight and reporting obligations under U.S. federal securities laws and the scrutiny of our investors and these obligations could divert their attention away from the management of our business, which could affect our business, financial condition,results of operations and prospects.
Risks Associated with Common Stock and Other Securities
•It is not currently anticipated that we will pay dividends on shares of common stock, and, consequently, your ability to achieve a return on your investment will depend on appreciation, if any, in the market price of common stock.
•Future sales of common stock may depress their stock price.
•We could become subject to product liability claims, product recalls, and warranty claims that could be expensive, divert management’s attention and harm our business reputation and financial results.
•The public warrants may never be in the money, and they may expire worthless and the terms of the warrants may be amended in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment.
•We may redeem unexpired public warrants prior to their exercise at a time that is disadvantageous to the warrant holder, thereby making the public warrants worthless.
•We may issue additional shares of common stock or preferred stock, including under our equity incentive plan. Any such issuances would dilute the interest of our stockholders and likely present other risks.
•Our common stock will no longer be listed on the Nasdaq Global Market which may adversely affect our ability to raise additional financing through the public or private sale of equity securities, the ability of investors to trade our securities and the value and liquidity of our common stock.
•Our common stock is now quoted on the OTC Markets OTCQB Venture Market tier which is a volatile market that could depress the market price of our common stock and have an adverse impact on our ability to raise capital in the future for reasons unrelated to our operating performance.
v
Part I
Item 1: Business
The following discussion reflects the business of the Company. In this section, “we,” “our,” “the Company” or “QT Imaging” below generally refers to QT Imaging Holdings, Inc. and its subsidiaries.
Background of the Business Combination
On December 8, 2022, GigCapital5, Inc. (“GigCapital5”), our legal predecessor company and special purpose acquisition company, which was incorporated in Delaware in 2021, entered into a business combination agreement (“Business Combination Agreement”) with QTI Merger Sub, Inc., a subsidiary of GigCapital5 (“Merger Sub”) and QT Imaging, Inc. When the transactions contemplated under the Business Combination Agreement closed on March 4, 2024 (the “Business Combination”), Merger Sub merged with and into QT Imaging, Inc., with QT Imaging, Inc. the surviving company in the Business Combination, and after giving effect to the Business Combination, QT Imaging, Inc. became a wholly owned subsidiary of GigCapital5, which was renamed as QT Imaging Holdings, Inc.
QT Imaging, Inc. was incorporated in Delaware on December 31, 2020 by converting from its predecessor, QT Ultrasound LLC (“QT Ultrasound”), which was a limited liability company formed in 2012 to focus on the research, development, and commercialization of innovative body imaging systems using low energy sound. On that date, QT Imaging, Inc. changed its name from QT Ultrasound LLC to QT Imaging, Inc. and revoked the limited liability company election status by the incorporation.
QT Imaging Holdings, Inc. is headquartered in Novato, California.
Overview
A Novel Body Imaging Technology
The Company—with the support of nearly $181 million in financial support from the U.S. National Institutes of Health—has developed a novel, comprehensive body imaging technology that has high resolution, high sensitivity, high specificity, high positive and negative predictive values and is safe and inexpensive. The technology is based on ultra-low frequency transmitted sound and uses a one-of-a-kind novel sound back-scatter design and inverse-scattering reconstruction to create its images.
The Company is a medical device company founded in 2012 and engaged in the research, development, and commercialization of innovative body imaging systems using low energy sound. We believe that medical imaging is critical to the detection, diagnosis, and treatment of disease and that it should be safe, affordable and accessible. Our goal is to improve global health outcomes through the development and commercialization of imaging devices that address critical healthcare challenges with accuracy and precision.
The current standard of care for imaging in breast cancer screening, diagnosis, and treatment is far from satisfactory. Generally, the process starts with X-ray mammography, the primary screening tool for women. Mammography uses radiation, which in sufficient cumulative doses can increase the risk of cancer; is uncomfortable to painful for patients as it involves breast compression. Callbacks for adjunct screening and diagnosis include ultrasound, magnetic resonance imaging (“MRI”), and may include biopsies. This process is expensive, time consuming, and can be trying for women. Also, the use of three imaging modalities in the process speaks to the weakness of any one in adequately screening for breast cancer.
The Company’s opportunity in breast imaging is to speed the time to diagnosis for women with cancer, and to provide assurance for women who do not have the disease with a better patient experience and lower cost than the current standard of care.
The current QT Breast Scanner developed by the Company is a Class II device subject to premarket notification and clearance under Section 510(k) of the Federal Food, Drugs, and Cosmetics Act (“FDCA”). On August 23, 2016, QT Ultrasound submitted a Section 510(K) Summary of Safety and Effectiveness application for the QT Breast Scanner in accordance with 21 CFR 807.92 under 510(K) Number K162372. As part of meeting the general requirements for basic safety and essential performance of the QT Breast Scanner (formerly, QT Ultrasound Breast Scanner) pursuant to AAMI
1 This is comprised of previous grants including grants to the University of Utah ($811,000) and the current five year grant 1RO1CA273700 from the U.S. National Institute of Health for $2.58 million (Quantitative Ultrasound Monitoring of Breast Cancer Therapies), which was awarded to 3 institutions: University of Illinois, University of Toronto (Sunnybrook) and QT Imaging, which received $1.08 million of the $2.58 million grant.
1
ES60601-1:2005/(R)2012 and A1:2012 Medical electrical equipment, testing was conducted by Intertek, an independent testing laboratory, located in Menlo Park, CA. Intertek also conducted applicable testing pursuant to IEC 60601-1-6 Edition 3.1 2013-10-Medical electrical equipment Part 1-6 General requirements for safety—Collateral Standard: Usability. In addition, QT Ultrasound conducted, and Intertek witnessed, all applicable testing pertaining to the requirements for the safety of ultrasonic medical diagnostic and monitoring equipment and to demonstrate compliance with the “Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment”. This test on acoustic output was pursuant to IEC 60601-2-37 Edition 2.0.2007 Medical electrical equipment—Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment. Finally, system verification testing was conducted to ensure that the QT Breast Scanner met all design and other requirements including but not limited to that no new issues of safety or effectiveness compared to the predicate device, SoftVue System manufactured by Delphinus Medical Technologies, were raised.
On June 6, 2017, the FDA, in response to the Company’s Section 510(K) Summary of Safety and Effectiveness premarket notification, determined that the QT Breast Scanner is substantially equivalent to the predicate device. Our use of the words “safe”, “safety”, “effectiveness”, and “efficacy” in relation to the QT Breast Scanner in this annual report and all other documents related to the Company is limited to the context of the Section 510(K) Summary of Safety and Effectiveness that was reviewed and responded to by the FDA.
The Cost and Accessibility of Healthcare
Medical imaging is an essential part of clinical diagnosis and is a requirement for making the best treatment decisions and improving a person’s health. Most people in the world live in low-resource environments and do not have access to advanced medical imaging—thus the absence of high-quality medical imaging in low resource environments (“LREs”) is a significant obstacle to providing basic health care.
Even in advanced health care facilities in the U.S., where adequate medical imaging is available, the cost of this medical imaging is very high—driving up healthcare costs and limiting accessibility to many people with limited income, high insurance deductibles or those in LREs or rural areas.
The Purpose of the Company
Most conventional imaging technologies—X-ray computed tomography (“CT”), MRI and positron-emission tomography (“PET”)—used in tertiary care require high energy, protective shielding of the patient, trained medical staff to operate the equipment, the administration of chemical agents to the patient to increase contrast and optimize visualization and specialized trained technicians to operate the equipment and ensure patient safety. Furthermore, the imaging procedures using these technologies are cumbersome, time-consuming and expensive. In addition, these conventional imaging technologies or modalities are not amenable to direct-to-consumer (“DTC”) or point-of-care (“POC”) settings or available in LRE. The Company believes that its new technology can address the issues presented by these conventional imaging technologies with its accurate, safe, less expensive and easily deployable imaging systems.
The Clinical Problem
The current medical imaging technologies—CT, MRI and PET—are commonly used in advanced health care facilities in North America, Europe, Japan and South Korea, with more limited deployment in selected tertiary care facilities in other countries—usually large urban areas. These current technologies are based on advanced engineering solutions that use high energy (X-rays, positrons or nuclear magnetic resonance signals) to see inside of the human body. These technologies require large capital investments, are limited to specialized facilities and require advanced certifications for the machines and their operators to insure safe operation. These machines are also expensive to purchase and maintain. All these factors combine to restrict their deployment to advanced clinical centers and tertiary care institutions.
A Solution to Increasing the Quality of Health Care and Lowering Costs
Advances in technology offer an opportunity to provide: 1) a means for obtaining better image quality in medical images, 2) access to DTC or direct to practitioner (“DTP”) medical imaging, 3) lower cost medical imaging, 4) reduced inconvenience and risk for patients by providing a safe alternative to high-energy imaging and 4) a lower cost solution for making a medical diagnosis. The Company believes that its technology is ideal for DTC, DTP and POC use because of its high performance, safety and relatively low cost. Furthermore, we believe that providing increased patient access to safe medical imaging is one important solution to increasing access and lowering the costs of medical care.
The Company intends to follow a staged entry into the market, beginning with clearances already in place which support its use as an adjunct to mammography and to monitor treatment. Additionally, the Company intends to pursue a
2
regulatory and commercial pathway to support the use of its technology as an alternative to breast MRI in screening younger women (under 40 years of age) at above-average risk for breast cancer. In parallel, the Company will then use these placements as footholds from which to build presence in the medical community and acquire the data needed for additional FDA clearances and insurance reimbursement. In parallel with these efforts, the Company solidified existing business and distribution strategic partnerships to cover sales in US, as well as partnerships to build strong manufacturing processes to deliver the QT Breast Scanners in large scale production.
Our Competitive Strengths
We believe that our competitive strengths include the following:
•The world-wide market for medical imaging is large and it has a potential to expand in the areas where the Company has differentiation;
•a non-ionizing, non-contrast dye injection imaging modality;
•an imaging modality with superior performance as compared to traditional mammogram with respect to specificity (false positive), thus less unnecessary emotional trauma for patients, reduced numbers of invasive follow-up procedures and a reduction of costs for both patient and broader society;
•an imaging modality with the potential to offer superior specificity compared to screening breast MRI for younger women (under 40 years of age) at above-average risk. By reducing false positives, it holds the potential of sparing patients from undue emotional distress, limiting unnecessary biopsies and follow-up procedures, and ultimately decreasing healthcare costs for both individuals and society;
•a lower price point than conventional high-energy imaging equipment;
•the Company’s technology can be deployed to LREs because of its automation, small footprint, no shielding, no contrast-dye injection;
•the Company’s technology is portable and can be used in POC settings such as LREs;
•the Company’s technology is deployable in outdoor settings such as sports, military, and naval settings;
•the Company’s technology reduces the barriers to testing and follow up-care for women, as there is no need for specialized training and the technology is well-suited for lowering health care costs by being affordable and easily accessed;
•the Company’s technology provides optimized patient experience, as no radiation is involved, with the patient being able to be followed with no limitation to imaging frequency;
•the Company’s technology is well-suited for traditional tertiary care hospitals and additionally for DTC and DTP applications, that are outside these institutions;
•the Company’s technology is uniquely proprietary, disruptive and a one-of-a kind product that can address a variety of unmet medical needs in the medical marketplace;
•the Company’s scanner features a uniquely simple design with a small number of components, which in turn significantly reduces the cost of the bill-of-materials (“BOM”), cost-of-good-sold (“COGS”), and the total-cost-of-ownership, and enables a lower average sales price (“ASP”) compared to other available systems, thus making it much more affordable to large mass deployments; and
•the Company’s products have potential strong revenue growth, with capital purchase supporting substantial long-term gross margin.
Our Strategies
We believe that our strategies include the following:
•Create disruptive technological innovation (software, artificial intelligence, and smart physics) to improve medical imaging and thus health care quality and access.
3
•Continue to improve our high quality, high resolution, native 3D, reproducible image quality regardless of operator or breast size/tissue type breast imaging technology, as well as the techniques for quantifiable analysis, comparison, and training.
•Partner with strategic business and distribution channels to address the U.S. market for breast imaging im-mediately and, other regions in the future, to place the QT Breast Scanner in hospitals, radiology centers, etc. and generate awareness of the benefits of the Company’s technology.
•Perform small scale manufacturing internally to the Company and partner strategically for large scale manufacturing.
•Expand the market by supporting additional DTC and DTP approaches to enable the ability to lower health care costs and increase access via personal medical imaging.
•Provide a new social and economic opportunity for consumers to take control of some aspects of their own health care—such as imaging for minor injuries or medical conditions without needing a healthcare “gate-keeper.”
•Focus our intellectual capabilities and ethical framework to become unified in our mission to improve the quality and lower the cost of health care world-wide... “It’s about time.”
•Leverage on the intellectual property and know-how of the company as is demonstrated through the first family of QT Breast Scanner commercialization, to develop other scanning medical imaging products, such as infant scanners, full-body scanner and/or other products.
Industry & Market Opportunity
Doctors and hospitals are increasingly turning to medical imaging to screen for and diagnose cancer, support and monitor ongoing cancer treatment (drugs, radiation, and surgery), and offer non-invasive surgical options for patients. This has resulted in a major market opportunity—the annual worldwide medical imaging market currently is estimated to be $40 billion, with $10 billion coming from the United States.2 Global cancer screening, with an approximately $150 billion market size in 2022, is expected to grow at a compound annual growth (“CAGR”) of 12% and reach approximately $472 billion in 2033.3
Breast Imaging
Breast cancer detection and diagnostic technologies (including mammography, MRI, and ultrasound, as well as genetic testing and image guided breast biopsy) are a significant part of the medical imaging market and are estimated to represent a $4.6 billion global market in 2023 with an ongoing CAGR of 8%.4 The market is segmented by technology, primarily between ionizing breast imaging (e.g., mammography) and non-ionizing breast imaging, which includes ultrasound and MRI. The non-ionizing segment is expected to grow at a faster rate5 than the ionizing segment due to technological advances such as better segmentation of anatomical detail, higher sensitivity to small breast lesions in women with dense breast tissue6, and fewer false positives.
2 See, Fortune Business Insight, Medical Imaging Market Size, Share & COVID-19 Impact Analysis, Type (Magnetic Resonance Imaging, Computer Tomography, X-ray, Ultrasound, and Molecular Imaging), By Application (Cardiology, Neurology, Orthopedics, Gynecology, Oncology, and Others), by End User (Hospitals, Specialty Clinics, Diagnostic Imaging Centers, and Others), and Regional Forecast, 2021-2028 (Jan. 2022), available at https://www.fortunebusinessinsights.com/industry-reports/medical-imaging-equipment-market-100382.
3 See, Fortune Business Insight, Medical Imaging Market Size, Share & COVID-19 Impact Analysis, Type (Magnetic Resonance Imaging, Computer Tomography, X-ray, Ultrasound, and Molecular Imaging), By Application (Cardiology, Neurology, Orthopedics, Gynecology, Oncology, and Others), by End User (Hospitals, Specialty Clinics, Diagnostic Imaging Centers, and Others), and Regional Forecast, 2021-2028 (Jan. 2022), available at https://www.fortunebusinessinsights.com/industry-reports/medical-imaging-equipment-market-100382.
4 See, ReportLinker, Global Breast Imaging Technologies Market to Reach $5.8 Billion by 2030 (Feb. 2, 2023), available at https://finance.yahoo.com/news/global-breast-imaging-technologies-market-192600736.html?guccounter=1&guce_referrer=aHR0cHM6Ly9kdWNrZHVja2dvLmNvbS8&guce_referrer _sig=AQAAAJGTUiAxSng9741aF3B3-AT5uFrtLSoqRIo_b38QWPbYdAjvx0aejvhKoF-p3Yvh4jZ41GAPV6VDMPuYjtfUAHqMdEQhdA5buqzcGISJDID04pNvYySjQ92AlaTPNAa99CWcRem UxEbDmGEKPetyTskvCpwcWKRA8ZIWA_2Nb3mh.
5 See, GrandViewResearch, Magnetic Resonance Imaging Market Size, Share & Trends Analysis by Architecture, by Field Strength, by Application (Brain & Neurological, Vascular), by End Use, by Region, and Segment Forecasts, 2022-2030, available at https://www.grandviewresearch.com/industry-analysis/magnetic-resonance-imaging-market (last visited Feb. 10, 2023).
6 NIH, National Cancer Institute, Dense Breasts: Answers to Commonly Asked Questions (“Breasts contain glandular tissue, fibrous connective tissue, and fatty breast tissue. Breast density is a term that describes the relative amount of these different types of breast tissue as seen on a mammogram. Dense breast tissue has relatively high amounts of glandular tissue and fibrous connective tissue and relatively low amounts of fatty breast tissue.”), available at https://www.cancer.gov/types/breast/breast-changes/dense-breasts (Mar. 29, 2023).
4
The current standard of care for imaging in breast cancer screening, diagnosis, and treatment is far from satisfactory and may involve certain side effects. There are adverse effects to the use of medical imaging methods such as ionizing radiation, mammography, and MRI. Generally, the process starts with X-ray mammography, the primary screening tool for women. Mammography uses radiation, which in sufficient cumulative doses can increase the risk of cancer; and is uncomfortable or too painful for patients as it involves breast compression. Another adverse effect is the inefficiency of mammography in detection of cancer in women with dense breasts. There is a psychological adverse effect of callbacks for adjunct screening and diagnosis include ultrasound, MRI, and may include biopsies. This process is expensive, time consuming, and can be mentally and physically trying for women. Also, the use of three imaging modalities in the process speaks to the weakness of any one in adequately screening for breast cancer. MRI may take place in a closed environment which may cause claustrophobia, require sedation or general anesthesia and may require injection of a heavy-metal contrast agent. The use of sedation or anesthetic drugs risks severe compromise of respiratory and cardiac function.
Regarding CT, the harmful effects of radiation used in ionizing radiation exposure raises the risk of cancer, including leukemia, breast cancer, thyroid cancer and brain cancer. These issues make MRI preferred to CT for all but trauma evaluation.
The QT Breast Scanner has no reports of adverse effects from the more than 20,000 scans performed to date. Similar to other ultrasound devices, due to the low frequency and energy of those scanners, including handheld devices, there are no known significant risks reported in general clinical practice. The QT Breast Scanner does not require potentially harmful ionizing radiation or anesthesia and is done in an open environment thereby decreasing stress and the necessity of sedation. As a result, there is the potential for increased imaging efficacy. The QT Breast Scanner does not cause breast implant displacement or rupture.
The Company’s opportunity in breast imaging is to speed the time to diagnosis for women with cancer, and to provide assurance for women who do not have the disease with a better patient experience and lower cost than the current standard of care.
While the Company believes women will embrace its technology, the Company is focused on achieving clinical adoption through the medical community, which requires continuing research on the clinical efficacy of the images rendered by the QT Breast Scanner known as a QTscan® image and development of key opinion leaders (“KOLs”) who can speak to the clinical value of its machines in practice. In addition, the Company must navigate the economics and price controls of the U.S. reimbursement system, as well as the economics and price controls of any foreign country in which the Company’s products and product candidates may receive regulatory approval. The Company is also actively working to expand its regulatory clearances to support marketing the QT Breast Scanner as an alternative to breast MRI for screening, initially targeting above average-risk younger women and ultimately aiming to provide a screening option for all women. Finally, even given the achievement of all of these objectives, there must be a “critical mass” of installed scanners –patients must be able to access the machines.
There are three primary technologies within the non-ionizing breast imaging segment: Automated Breast Ultrasound Systems or ABUS; Breast Ultrasound Tomography Systems; and Photoacoustic Imaging.
Automated Breast Ultrasound Systems (“ABUS”)
The ABUS segment is the largest and is expected to grow at a CAGR of 16% worldwide over the next five years, with more than 2,000 installations in place and a market value of $850 million by 2024.7 This growth will be driven by the advantages inherent in ABUS: quick turnaround time, affordability of devices, ease of device deployment, accurate diagnostic results, and operations without continuous operator monitoring. In addition, contextual factors including rising health awareness, government advocacy for breast cancer awareness, and an increasing prevalence of breast cancer will contribute to the expansion of this market.
ABUS technologies typically use a reflection transducer8 (5-15 MHz) and not transmitting setup, in a “motorized” arrangement. The major developers of such systems include: 1) the Acuson S2000 ABVS (sold by Siemens); 2) the Invenia System (s
7See, MarketResearch, Automated Breast Ultrasound System Market Size Outlook in 2023 and Beyond: Market Trends, Insight, Growth Opportunities, Market Share and Forecasts by Types, Applications, Countries and Companies to 2023 (Feb. 2023), available at https://www.marketresearch.com/VPA-Research-v4245/Automated-Breast-Ultrasound-System-Size-33347813/.
8 A medical reflection transducer, also known as an ultrasound transducer, is a device that converts electrical energy into sound waves, and the back again into electrical energy. It is used in medical imaging to produce images of internal organs and tissues in the body, and it is used in various medical imaging techniques such as ultrasound, echocardiography and Doppler imaging. See, e.g., ECG & ECHO Learning, The Ultrasound Transducer, available at https://ecgwaves.com/topic/the-ultrasound-transmitter-probe/ (last visited Apr. 4, 2023); see also, FDA, Ultrasound Imaging Sept. 28, 2022), https://www.fda.gov/radiation-emitting-products/medical-imaging/ultrasound-imaging.
5
old by GE Healthcare); 3) a manual video loop AWBS System (sold by Sono-Cine); and 4) a motorized single transducer Sofia System (sold by Hitachi.). All of these systems produce B-mode reflection images9.
Breast Ultrasound Tomography Systems
These technologies use traditional reflection transducers10 in an “array” configuration around the breast: 1) Mastoscopia (Greece); 2) the KIT system (research only) from Karlsruhe University in Germany; and 3) the Delphinus System.
Photoacoustic Imaging
Photoacoustic imaging systems utilize lasers to excite tissues and produce acoustic energy that subsequently create images of the breast vasculature.11 Such systems include both photoacoustic tomography (“PAT”) and photoacoustic imaging (“PAI”) systems. While the PAT systems allow volumetric imaging by reconstructing stacks of 2D images, the PAI systems only allow superimposition of photoacoustic signal information on top of conventional B-mode ultrasound. Note that in comparison to ultrasound, photoacoustic imaging systems inherently lack the ability to image the tissue anatomy and essentially only image the vasculature (i.e., blood, which is a strong absorber of light). For PAT systems, there is no clinical trial data available (to our knowledge) and no PAT systems have been approved for clinical use. For PAI systems, the Imagio Breast Imaging has been used in clinical trials and is FDA cleared to be used as an adjunct to conventional handheld breast ultrasound.
All of these technologies face challenges to expansion, including FDA clearances and insurance reimbursement. However, the shortcomings of other imaging methods such as ionized radiation exposure, high costs and deployment challenges of MRI, and the inefficiency of mammography in detection of cancer in women with dense breasts, are sufficiently compelling for industry to address these obstacles, and these shortcomings will continue to create an opportunity for development and commercialization of advanced screening systems such as the Company’s.
To our knowledge at the time of filing this annual report, we are not aware of any technologies approved for primary screening clearance by the FDA except for various types of technology related to X-ray mammography.
Future Market Opportunities
While the Company’s short-to-medium term focus will be on breast scanning, future products will open additional markets. The Company’s Open Partial Angle Scanner concept, which is currently under development, is expected to provide entry into the global orthopedic medical imaging market, which is estimated to be $7.3 billion by 202512.
9 B-mode ultrasound, also known as 2D ultrasound, is a type of ultrasound imaging where “a linear array of transducers simultaneously scans a plane through the body that can be viewed as a two-dimensional image on screen.” See, NIH, National Library of Medicine, Carovac A., Smajlovic F., Junuzovic D., Application of Ultrasound in Medicine, 19(3) Acta Inform Med. 168-171 (Sept. 2011), available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3564184/.
10 Medical reflection transducer, also known as an ultrasound transducer, is a device that converts electrical energy into sound waves, and the back again into electrical energy. It is used in medical imaging to produce images of internal organs and tissues in the body, and it is used in various medical imaging techniques such as ultrasound, echocardiography and Doppler imaging. See, e.g., ECG & ECHO Learning, The Ultrasound Transducer, available at https://ecgwaves.com/topic/the-ultrasound-transmitter-probe/ (last visited Apr. 4, 2023); see also, FDA, Ultrasound Imaging Sept. 28, 2022), https://www.fda.gov/radiation-emitting-products/medical-imaging/ultrasound-imaging.
11 Breast vasculature refers to the blood vessels that supply and drain blood from the breast tissue. The breast is a highly vascularized organ, and the blood supply comes from a network of arteries and veins that run throughout the breast tissue. See, NIH, National Library of Medicine, Yusuf S. Khan, Hussain Sajjad, Anatomy, Thorax, Mammary Gland, available at https://www.ncbi.nlm.nih.gov/books/NBK547666/ (last updated July 25, 2022).
12 See, MarketWatch, Orthopedic Medical Imaging Market Size Analysis between Two International Players through Business Aspects Way 2026 (Feb. 6, 2023), available at https://www.marketwatch.com/press -release/orthopedic-medical-imaging-market-size-analysis-between-top-international-players-through -business-aspects-way-2026-2023-02-06.
6
Company’s Products & Product Road Map
Current Products

Proposed Products Under Development

QT Breast Scanner
The Need: A safe, painless imaging device that provides conclusive breast health assessment
Background:
Breast cancer is the most commonly diagnosed women’s cancer in the United States, according to the National Cancer Institute. The American Cancer Society estimates that in 2022, 287,850 women in the United States have been diagnosed with invasive and in situ (early stage) breast cancer, and breast cancer has claimed the lives of 40,920 women.13 The American Cancer Society further estimates that one out of every eight women will develop breast cancer at some point during her life and one in every 42 women who turns 50 today will have a diagnosis of breast cancer before she turns 60.
There are several dominant screening and diagnostic technologies that are used both independently and dependently to locate cancers at an early stage and improve treatment outcomes. Each of the currently available non-surgical modalities for breast cancer detection has various clinical limitations. Screening methods and technologies include: (i) breast self-
13 See, American Cancer Society, Breast Cancer Facts & Figures, available at https://www.cancer.org/research/cancer-facts-statistics/breast-cancer-facts-figures.html (last visited Feb. 10, 2023).
7
examination and clinical breast examination; (ii) mammography, including screening mammography, diagnostic mammography, and mammography with computer aided detection; (iii) Hand-Held Ultrasound (“HHUS”) and (iv) MRI.
Mammography is the dominant imaging modality in today’s standard of care. The American Cancer Society recommends that women of average risk have the option to begin mammography at age 40, get mammograms every year from age 45-54, and have mammography every other year starting at age 55.14 Despite those recommendations, only 65% of women over 40 in the United States have had a mammogram in the previous two years, and only 58% of women between 40 and 49 had a mammogram in the previous two years,15 even though screening mammography is 100% covered under the Affordable Care Act. This is in part due to the limitations of mammography, both in terms of sensitivity and reliability for dense breast tissue, where 10-15% of cases have inconclusive results requiring further testing, as well as concerns about safety. Mammography is also problematic in women who have breast implants. For these women, problems include painful mammograms, delayed detection of cancer from interference in imaging breast tissue, and an unwillingness to perform mammograms due to fear of implant rupture, dislocation or capsular contracture.
The Company’s goal is to provide highly accurate, 100% safe, radiation-free and painless breast imaging that can be used to:
1)identify cancer early to minimize invasiveness and increase effectiveness of treatment; and
2)eliminate unnecessary intervention (additional imaging and biopsies) for women with benign breast conditions, most notably cysts.
The Product, QT Breast Scanner
The QT Breast Scanner is a fixed, stationary, mechanical scanner used to evaluate the breast without the use of either ionizing radiation or compression associated with mammography, or the injections required for breast MRI. With the QT Breast Scanner, the patient lies comfortably on a table which contains an opening through which the breast is immersed in a warm water bath (see Image 1) and gently immobilized using a magnetic retention pad fixed to a magnetic rod.
The QT Breast Scanner

Image 1
Surrounding the warm water bath is a dual modality reflection and transmission ultrasound array that rotates 360 degrees around the breast (see Image 2 & Image 3) to produce 3D images. The ultrasound array produces low energy, low frequency sound waves (non-radiation “pressure waves”) through the breast and reflecting from the breast, with both collecting volumetric (3D) data. Reflection data is collected on the transducer facing side, and the transmission data is collected on the back side of the breast. The transmission data quantitatively measures the velocity of these pressure waves through the breast. This information can be used to generate a true 3D image of the breast and all its tissues. The QT Breast Scanner differs from the handheld ultrasound used in breast imaging in that it utilizes reflection and transmission data from low-frequency sound waves, providing a significant increase in diagnostic information using the speed of sound characteristics of the breast and acquiring in true 3D a very accurate rendering of the breast tissue. The QT Breast Scanner provides sub-millimeter, high-definition, image resolution enabling identification of normal and abnormal breast structures and the accurate depiction of the precise shape and location of findings. The technology uniquely quantifies breast density using transmission information to further personalize a patient’s management recommendations. Surface-to-volume ratios
14 See, American Cancer Society, American Cancer Society Recommendations for the Early Detection of Breast Cancer, available at https://www.cancer.org/content/cancer/en/research/infographics-gallery/breast-cancer-screening-guideline.html (last visited Feb. 10, 2023).
15 U.S. Department of Health and Human Services, “Health, United States, 2016”, Table 70.
8
and volumetric doubling time growth rate characteristics can be calculated to determine significance of lesions and improve specificity of the ultrasound.
 The transducer array in the water bath Image 2 |  Schematic of rotating ultrasound transmitter Image 3 | |||||||
The QT Breast Scanner creates true 3D images of the patient’s breast viewable in the Quantitative Transmission Ultrasound Viewer (known as QTviewer®), a software product designed for healthcare professionals to view the transmission (speed of sound) and reflection images. This application can display correlated Digital Imaging and Communications in Medicine (“DICOM®”) images in multiple orientations (coronal, sagittal, and axial). QTviewer can manipulate image views and analyze pixel data with various functions. The QTviewer has additional functionality which enables the user to measure mass size and volume as well as fibroglandular tissue volume.16
Image 4 below is a still image of the viewer for a patient with a cyst. The transmission (top 3 panels) and reflection (bottom 3 panels) images as seen in coronal, axial, and sagittal representations.

Image 4
The QT Breast Scanner is the current version of the QT Breast Scanner and is FDA-cleared “for use as an ultrasonic imaging system to provide reflection-mode and transmission-mode images of a patient’s breast. The device is not intended to be used as a replacement for screening mammography.”17
16 See, American Association for Cancer Research, R. Natesan, J. Wiskin, S. Lee, B. H. Malik, Quantitative Assessment of Breast Density: Transmission Ultrasound is Comparable to Mammography with Tomosynthesis (Dec. 3, 2019), available at https://aacrjournals.org/cancerpreventionresearch/article/12/12/871/47203/Quantitative-Assessment-of-Breast-Density.
17 U.S Department of Health and Human Services, Food and Drug Administration, 510(k) number K162372.
9
The QT Breast Scanner has current applicability as a supplementary imaging device (not as a replacement for screening mammography); near-term applicability for determining breast density, measuring mass size and growth, and diagnosing lesions using artificial intelligence; and medium- to long-term applicability for breast screening as shown in Table 1.
| Use of the QT Breast Scanner | Value it Adds | QT Timeframe* | ||||||||||||
| Supplementary imaging | Adjunct to screening mammography (not a replacement), particularly for women with dense breasts to identify masses missed by mammography or provide additional information on masses seen, with the potential to reduce unnecessary procedures | Current | ||||||||||||
| Fibroglandular Tissue Volume & the Ratio of Fibroglandular Tissue Volume to Total Breast Volume | Ability to quantify this ratio (a risk factor for breast cancer), without compression or radiation of mammography | Current | ||||||||||||
| Mass Size and Growth | Ability to measure response to treatment and assess mass stability | Short term | ||||||||||||
| A.I.-Based Mass Diagnostics | Reduce unnecessary procedures (biopsies, additional imaging) by identifying lesion type | Short term | ||||||||||||
| Screening for High-Risk Young Women | Provide young women a safe, comfortable, accurate method to screen for breast cancer, as an alternative for breast MRI | Medium term | ||||||||||||
| Table 1 | ||||||||||||||
__________________
*Note: the foregoing is based on the Company’s current estimates and the timeframe is subject to change due to various factors, including those described in the “Risk Factors” section and elsewhere in this annual report.
Breast Scanner Clinical Images
The images below (Image 5 and Image 6) compare an artist’s depiction of the normal breast anatomic features (top) and QTscan® images rendered by the QT Breast Scanner of a normal human breast (below) showing the skin, fat, breast duct and glandular (terminal) units of the living breast. The Cooper’s ligaments, ducts, and glandular structures are not visible in conventional breast screening imaging.
Schematic anatomy of the breast

10
Image 5

Transmission (left) and reflection tomograms of the breast. The white and black squares in the speed of sound image (left) mark fat and glandular tissue, respectively. Single and double black arrows mark ductal tissue and skin, respectively. Reflection image (right): Single white arrows mark the connective tissue identified as Cooper’s ligaments.
Image 6
Two key metrics in breast imaging are sensitivity and specificity. Mammography has well-recognized challenges with sensitivity in dense breasts. Image 7 below compares the same breast across different imaging modalities. In addition to demonstrating differences in image quality across modalities, it represents a case where a mass was not visible on mammography but is visible on the MRI and QTscan.

Image 7
The Company recently conducted a mini study looking specifically at the ability of its technology to identify masses in dense breasts compared to mammography. Forty cases were selected in which there was a finding on the QTscan. The cases were selected from a “Case Collection Study to Determine the Accuracy, Call Back and Cancer Detection Rates of QT Ultrasound in Breast Imaging (ACCRUE)” sponsored by QT Ultrasound LLC. This is available at ClinicalTrials.gov Identifier: NCT03052166. Two cases were subsequently excluded as there was not a corresponding mammogram, leaving 38 cases for comparison. The ACCRUE study was a prospective, multicenter, multi-arm case collection study which followed an adaptive design with an initially planned total enrollment of approximately 600 cases to include both benign and malignant cases, representative of all tissue densities. The study type was observational with an actual enrolment of 755 participants starting in April 2017. The end date was initial December 31, 2019, but actual completion date was January 1, 2020. There were three cohort groups: Cohort A-The group of asymptomatic subjects who have been given BI-RADS 1 or 2 based on their most recent standard of care assessment. All subjects received a QT Ultrasound scan. Cohort B-The group of asymptomatic women who have been given BIRADS categories 4 or 4a, 4b, 4c or 5 based on their most recent standard of care assessment. All subjects received a QT Ultrasound scan. Cohort C -The group of women who have been given BI-RADS categories 1, 2, 3, 4 or (4a, 4b, 4c), 5 or 6 based on their most recent standard of care assessment. All subjects received a QT Ultrasound scan. Subjects are assigned to Cohort C when it has been determined they cannot be assigned to Cohort A or Cohort B. The mammograms were interpreted by board-certified breast radiologists. In 32 of those cases, abnormalities identified using the QTscan were not identified on the mammogram. Image 8 (following) is one of
11
those cases, where a solid mass was identified on the QTscan but not visible on the mammogram. The scope, size and design of these clinical studies are conducted in accordance with the provisions of the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. In some instances, the Company or one of its affiliates sponsored or designed the clinical studies and the Company’s employees analyzed or authored the results, findings, or articles. This was an exploratory study of limited scope in order to determine if further studies were warranted. The study was not powered for statistical analysis, but provided information to support a broader study. The full studies are available at www.qtultrasound.com/dense-breast-mass/.

Example of a QTscan mass (right) not seen on x-ray mammography (left)
Image 8
Some of the following additional studies were not conducted by the Company or specifically related to the QTscan. However, the Company or one of its affiliates sponsored or designed the clinical studies or the Company’s employees analyzed or authored the results, findings, or articles which are generally applicable to the Company or the QTscan.
1.M.P. Andre, C Barker, N Sekhon, J Wiskin, D Borup, K. Callahan: Pre-clinical experience with full-wave inverse scattering for breast imaging: Sound speed sensitivity, Acoustical Imaging 29:73-80, Springer, Dordrecht, 2009.
The Andre et al study was a pilot clinical study not conducted by the Company and there is no comparator. The number of participants was fewer than 20. This was a registry study not a comparative trial. There were no serious adverse events and no statistical analysis was needed, since there was no comparator.
2.J. Wiskin; D. Borup; S. Johnson; M. Berggren; D. Robinson; J. Smith; J. Chen; Y. Parisky; John Klock, ‘Inverse scattering and refraction corrected reflection for breast cancer imaging’, Jan D’hooge; Stephen A. McAleavey, Eds., Proc. SPIE, 7629, 2010.
The Wiskin et al study was a pilot clinical study involving the participation of Dr. Klock. There is no comparator. The number of participants was fewer than 20. This was a registry study not a comparative trial. There were no serious adverse events and no statistical analysis was needed, since there was no comparator.
12
3.Pellegretti, P, S Dellepiane, M. Vicari, M. Zani, M. Weigel, D. Borup, J. Wiskin, U. Saueressig, E. Kotter, and M. Langer A Clinical Experience of a Prototype Automated Breast Ultrasound System Combining Transmission and Reflection 3D Imaging, UFFC 2011-IEEE International Ultasonics Symposium Oct. 18-21, 2011, Session P3Ab, b Tomography.
The Pellegretti et al study was a pilot clinical study not conducted by the Company and there is no comparator. The number of participants was fewer than 20. This was a registry study not a comparative trial. There were no serious adverse events and no statistical analysis was needed, since there was no comparator.
4.Andre, M. PhD, James Wiskin, PhD, Haydee Ojeda-Fournier, MD, Linda Olson, MD, David Borup, PhD, Melissa Ledgerwood, B.S., Steven Johnson, PhD, “Quantitative 3D Whole Breast Imaging with Transmission and Reflection Ultrasound” AAPM Ultrasound Imaging Symposium Breast Imaging and Guidance of Interventions
This Andre et al study was a pilot clinical study not conducted by the Company and there is no comparator. The number of participants was fewer than 20. This was a registry study not a comparative trial. There were no serious adverse events and no statistical analysis was needed, since there was no comparator.
5.J. Wiskin, D. Borup, K. Callahan, Y. Parisky, J. Smith, M. André, S. Johnson, Inverse scattering Results, Acoustical Imaging 30, pp. 61-68, Springer, Dordrecht, 2011.
This Wiskin et al study was a pilot clinical study not conducted by the Company and there is no comparator. The number of participants was fewer than 20. This was a registry study not a comparative trial. There were no serious adverse events and no statistical analysis was needed, since there was no comparator.
6.Andre, M, J. Wiskin et al., AIUM Annual Convention, New York, 2011, “Quantitative 3-Dimensional Whole-Breast Imaging With Transmission and Reflection Ultrasound”, Advanced Breast Imaging Symposium, Moderators: M. Andre and P. Carson, Ph.D.
This Andre et al study was a pilot clinical study not conducted by the Company and there is no comparator. The number of participants was fewer than 20. This was a registry study not a comparative trial. There were no serious adverse events and no statistical analysis was needed, since there was no comparator.
7.John C. Klock, Elaine Iuanow, Bilal Malik, Nancy A. Obuchowski, James Wiskin, and Mark Lenox. Anatomy-Correlated Breast Imaging and Visual Grading Analysis Using Quantitative Transmission Ultrasound. International Journal of Biomedical Imaging Volume 2016, Article ID 7570406, 9 pages http://dx.doi.org/10.1155/2016/7570406
This Klock et al study was a comparative clinical study of QT Breast Imaging vs standard X-ray mammography done at the request of the FDA, therefore it was not a clinical outcome study. The number of participants was fewer than 20. All images were anonymized. This was a registry study not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one requested by the FDA to see the trends-analysis of the QT versus the Mammography in identifying structures in the breast.
8.Bilal Malik Ph.D.*, John Klock M.D., James Wiskin Ph.D., and Mark Lenox Ph.D. Objective breast tissue image classification using Quantitative Transmission ultrasound tomography. Nature Sci. Rep. 6, 38857; doi: 10.1038/srep38857 (2016). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5146962/
This Malik et al study was a comparative clinical study of the Company vs standard tissue pathological analysis to determine the precise structure/image correlations of QT Breast Imaging. The study was not a clinical outcome study. The number of participants was fewer than 20. All images were anonymized. This was not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one to determine tissue pathology correlations with the QT images.
9.Elaine Iuanow, MD, Kathleen Smith, MBA, Nancy A. Obuchowski PhD†, Jennifer Bullen MS† and John C. Klock, MD. Accuracy of Cyst vs. Solid Diagnosis in the Breast Using Quantitative Transmission (QT) Ultrasound. Academic Radiology 2017 Vol 24:1148-1153; doi: 10.1016/j.acra.2017.03.024. Epub 2017 May 23; PubMed ID 28549870. Academic Radiology has posted the study in full for free. http://www.healthimaging.com/topics/womens-health/breast-imaging/and-coming-ultrasound-technology-shows-prowess-mammography-adjunct.
This Iuanow et al study was a comparative clinical study of the Company vs standard tissue biopsy analysis to validate the structure/image correlations of QT Breast Imaging, therefore it was not a clinical outcome study. The number of
13
participants was fewer than 20. All images were anonymized. This was not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one to determine tissue pathology correlations with the QT images.
10.John C Klock, Elaine Iuanow, Kathleen Smith, Nancy A and Obuchowski Visual Grading Assessment of Quantitative Transmission Ultrasound Compared to Digital X-ray Mammography and Hand-held Ultrasound in Identifying Ten Breast Anatomical Structures. BAOJ Clinical Trials 3: 015. (2017). https://bioaccent.org/clinical-trials/clinical-trials15.pdf
This Klock et al study was a comparative clinical study of QT Breast Imaging vs standard X-ray mammography and breast examination using handheld ultrasound done at the request of the FDA, therefore it was not a clinical outcome study. The number of participants was fewer than 20. All images were anonymized. This was a registry study not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one requested by the FDA to see the trends-analysis of the QT versus the Mammography in identifying structures in the breast.
11.Bilal Malik, Alyson Terry, John Klock and Mark Lenox. Sensitivity of Quantitative Transmission ultrasound to detection of microcalcifications. SPIE (International Society for Optics and Photonics) Meeting Houston Texas February 20, 2018.
This Malik et al study was a pilot clinical study involving the participation of Dr. Klock. There is no comparator. The number of participants was fewer than 20. All images were anonymized. This was a registry study not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one requested by the FDA to see the trends-analysis of the QT versus the Mammography in identifying structures in the breast.
12.Malik B, Klock JC. Breast Cyst Fluid Analysis Correlations with Speed of Sound Using Transmission Ultrasound , Academic Radiology 26:76-85, Jan 2019 https://www.sciencedirect.com/science/article/pii/S1076633218301788
This Malik et al study was a comparative clinical study of the Company vs standard tissue biopsy chemical and cytological analysis to validate the structure/image correlations of QT Breast Imaging, therefore it was not a clinical outcome study. The number of participants was fewer than 20. All images were anonymized. This was not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one to determine tissue pathology correlations with the QT images.
13.J Wiskin, B Malik, R Natesan, M Lenox. Quantitative Assessment of Breast Density Using Transmission Ultrasound Tomogaphy. Medical Physics VolXXX https://doi.org/10.1002/mp.13503
This Wiskin et al study was a comparative clinical study of the Company vs standard breast density measurements using X-ray mammography to validate the software used to determine breast fibroglandular volumes in women. It was not a clinical outcome study. The number of participants was fewer than 30. All images were anonymized. This was not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one to determine tissue pathology correlations with the QT images.
14.Natesan R, Wiskin JW, Lee S, Malik B. Quantitative assessment of breast density: transmission ultrasound is comparable to mammography with tomosynthesis. Cancer Prevention Research 12:871-826 2019. Doi: 10.1158/1940-6207.CAPR-19-068 https://cancerpreventionresearch.aacrjournals.org/content/early/2019/10/23/1940-6207.CAPR-19-0268
This Natesan et al study was a comparative clinical study of the Company vs standard breast density measurements using X-ray mammography to validate the software used to determine breast fibroglandular volumes in women. It was not a clinical outcome study. The number of participants was fewer than 50. All images were anonymized. This was not a comparative outcome trial of patient results. There were no serious adverse events and the statistical analysis was a Pearson Correlation Coefficient of QT fibroglandular volume versus the Volpara breast volume as shown below:
14

15.Wiskin, J., Malik, B., Borup, D. et al. Full wave 3D inverse scattering transmission ultrasound tomography in the presence of high contrast. Sci Rep 10, 20166 (2020). https://doi.org/10.1038/s41598-020-76754-3.
This Wiskin et al study was a comparative clinical study of the Company vs standard MRI imaging of human knees. to validate the performance of the Company to standard orthopedic MRI imaging. It was not a clinical outcome study. The number of participants was fewer than 30. All images were anonymized. This was not a comparative outcome trial of patient results. There were no serious adverse events and no statistical analysis was needed, since the study was a simple one to determine tissue pathology correlations with the QT images.
16.Wiskin J, Malik B, Ruoff C, Pirshaffiey N, Klock J. Whole body imaging using low frequency transmission ultrasound. Academic Radiology 2023 https://www.academicradiology.org/article/S1076-6332(23)00033-8/fulltext.
This Wiskin et al study was a comparative clinical study of the Company vs standard 3-Tesla MRI imaging of 4 neonatal piglets as surrogates for human newborn infants. This study was done to validate the performance of the Company to standard MRI imaging. It was not a clinical outcome study. This was a piglet study. No statistical analysis was needed, since the study was a simple one to determine tissue pathology correlations with the QT images and with MRI images.
17.Bilal Malik, PhD, Elaine Iuanow, MD, John Klock, MD. An Exploratory Multi-reader, Multi-case Study Comparing Transmission Ultrasound to Mammography on Recall Rates and Detection Rates for Breast Cancer Lesions. Academic Radiology Vol 29 – Supplement 1 S10-S18, Jan 1, 2022. doi:https://doi.org/10.1016/j.acra.2020.11.0.11 and https://www.academicradiology.org/article/S1076-6332(20)30646-2/fulltext
In this Malik et al study, three-dimensional Quantitative Transmission (“QT”) ultrasound imaging was used for the detection and diagnosis of breast cancer. QT ultrasound has high resolution and high contrast to noise ratio, making it effective in evaluating breast tissue. This study compared radiologists’ performance of noncancer recall rates and lesion detection rates using QT Ultrasound versus full-field digital mammography (“FFDM”) in a cross section of female subjects. In this multi-reader multi-case (“MRMC”) study, we examined retrospective data from two clinical trials conducted at five sites. All subjects received FFDM and QT scans within 90 days. Data were analyzed in a reader study with full factorial design involving 22 radiologists and 108 breast cases (42 normal, 39 pathology-confirmed benign, and 27 pathology-confirmed cancer cases). The main results used a random-reader random-case analysis adjusted for location bias performed after a primary predefined random-reader fixed-case analysis. The readers’ mean rate of detecting lesions of any type was 4% higher (p-value > 0.05) with the Company. The mean non-cancer recall rate improved significantly, showing a decrease of 16% with QT (p-value > 0.03), at the expense of a 2% decrease in the mean cancer recall rate (p-value > 0.05) in comparison to FFDM. Combining performance on cancer and noncancer recall rates, the mean area under the receiver operator curve of confidence scores improved significantly by 10% with QT (p-value = 0.01). This MRMC study indicated that QTscan improves non-cancer recall rates without substantially affecting cancer recall rates.
15
STATISTICAL ANALYSIS—The data were analyzed for the entire cohort of 108 breast cases (42 normal, 39 pathology-confirmed benign, and 27 pathology-confirmed cancer cases) using two general approaches: a random-reader fixed-cases (“RRFC”) analysis and a random-readers random cases (“RRRC”) analysis. RRFC analysis generalizes to the population of readers, but is specific to the particular case set and is termed random-reader fixed-cases analysis. In comparison, RRRC analysis generalizes both the case set and the population of readers. The RRRC analysis was expected to provide results more generalizable to new readers reading new cases, but with wider confidence levels compared to the RRFC analysis.
For both approaches, performance comparisons between QT and FFDM were summarized in terms of mean differences between readers and 95% confidence intervals (“CI”) for these differences with p-values determining the degree of statistical significance. The performance metrics included non-cancer and cancer recall rates and detection rates for all lesions. In addition, the study analyzed the mean area under the receiver operator curve (“ROC-AUC”) based on the readers’ confidence scores as a statistically efficient approach to evaluating the cancer and noncancer performance metrics combined into a single measurement. These analyses were performed according to the method of Obuchowski & Rockette with Hillis adjustment to the degrees of freedom. The RRRC analysis of ROC-AUC was performed with the software package ORDBM MRMC 2.5, written by Stephen L, Kevin M. Schwartz, and Kevin S. Berbaum. The trapezoidal/Wilcoxon method for curve fitting and jackknifing for the covariance estimation were used in the analysis. All other statistical analyses were performed in the statistical computing environment R version 3.4.0 or higher. No statistical adjustments were made for multiple analyses. The ground truth was established by one-year follow-up mammogram results for the normal cases and pathology results for the benign and cancer cases. All RRFC and RRRC results were adjusted post-hoc for location bias, considering recalls as correct only when the decisions were based on the correct ground-truth lesions. This adjustment is indicated because the severity of location bias is dissimilar for the two imaging modalities. Therefore, the study was adjusted for location bias to avoid favoring the modality with higher false-positive rates.
Leveraging the speed of sound attribute of transmission ultrasound, the QTscan offers advantages in specificity as well. Image 9 below compares the same breast on mammography and the QTscan. While the mass may be visible in mammography, mammography cannot be specific about whether the mass is malignant or benign. The QTscan identifies the mass as a benign cyst based on speed of sound as well as morphology.18

Example of a cyst visible on the QTscan (right) not sees on x-ray mammography (left)
Image 9
18 See, HealthImaging, Up-and-coming Ultrasound Technology Shows Prowess as Mammography Adjunct (May 24, 2017), available at https://healthimaging.com/topics/medical-imaging/womens-imaging/and-coming-ultrasound-technology-shows-prowess-mammography (providing a link to Academic Radiology, through which the study is accessible free of charge at https://www.academicradiology.org/article/S1076-6332(17)30207-6/fulltext).
16
Image 10 below is a case of lobular carcinoma. As with the previous cases, note that the QTscan offers comparable image quality and diagnostic information as an MRI, but without the high cost associated with MRI or patient experience issues associated with claustrophobia, radition or injection.

Example of a cancer not seen on x-ray mammography (far left), hand-held ultrasound (middle left) and non-enhancing on MRI with gadolinium injection (middle right) is clearly visible in the QTscan (far right).
Image 10
While the preceding cases describe and demonstrate certain advantages of the QTscan, due to limitations in print quality, case studies are best viewed on a high-quality monitor. Please visit the Company’s website to view additional case studies and image comparisons—https://www.qtimaging.com/casestudies/.
Comparison of the QT Breast Scanner with currently available devices19

19 A medical device articulating arm is a mechanical arm or support structure used in medical procedures to position or hold surgical instruments, cameras, or other medical equipment. The arm typically consists of several articulated segments or joints that can be adjusted and locked in place to achieve a specific position or orientation. Medical device articulating arms are commonly used in minimally invasive surgeries, such as laparoscopy or endoscopy, where precise control and positioning of instruments are essential for successful outcomes.
17
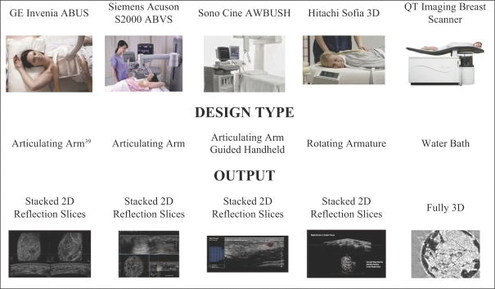

18

There are several differences between the QT Breast Scanner and HHUS, ABUS, BUST, PAI, and PAT devices.
Other devices use Piezo-electric transducers that provide primarily “B-mode” poor resolution data. There is no valid true “transmission mode” since they use shear wave. Their images have reflection and compounding artifacts. Furthermore, their images are compounded 2D slices and they do not acquire the data in 3D. The resolution of their “3D” mode, “speed” images and specificity for masses is poor and their contrast-to-noise ratios are low. Their images cannot differentiate calcifications so in our opinion at least 20% of all cancers, mainly DCIS and non-invasive cancers, are missed. They have no “functional” imaging features such as doubling time to diagnose slow-growing cancers, tissue identification and specific tissue volume segmentations. There is poor reproducibility of their measurement and volume data thus they cannot follow cancer treatments or do breast density measurements.
Very few companies undertake or sponsor comparative clinical trials and what data is produced lacks clinical usefulness in terms of sensitivity and specificity. Other than Delphinus’ secondary screening trial that we are aware of, many companies have failed to do head-to-head trials against mammography for primary screening. In their current iterations their technologies are not able to do body or orthopedic imaging for future growth and development.
Of critical importance in comparing the Company’s devices against other devices are factors such as their lack of FDA clearances for general screening, their lack of comparative trials for primary breast cancer screening, and the fact that their clinical resolution, presence of artifacts, and sensitivity and specificity data are not clinically useful.
Description of Future Products and Services
The Company believes that its Open Partial Angle Scanner concept, under development, will provide entry into the global orthopedic and infant medical imaging markets as described below. The following discussion and description of product candidates and their respective potential applications and uses is a discussion of the Company’s future products and product candidates, all of which are still in development stages and the Company can provide no assurance regarding when, if ever, these products and product candidates may be brought to market, or when, if ever, the Company would seek FDA premarket clearance or approval of a PMA application. As such, the discussion below contains information that is forward-looking in nature and investors are cautioned not to place undue reliance on these forward-looking statements.
Proposed QT Orthopedic Scanner
The Need—In-office orthopedic and extremity imaging joint and internal soft tissue diagnosis
Background:
The Company believes musculoskeletal conditions are the most common reasons for doctor visits, lost productivity, and disability in the United States. Among these, arthritis (osteoarthritis and rheumatoid arthritis) and back or spinal problems are the first and second leading causes of disability among adults. As the U.S. adult population ages, the prevalence of these conditions appears to be increasing, resulting in concomitant increases in healthcare resource utilization. According to the American Productivity Audit, pain of musculoskeletal origin (including back-pain, arthritis-related pain, and pain due to other musculoskeletal conditions) was reported by 7.2% of the workforce as having occurred over the previous two weeks.20 The knee is the most commonly injured joint by adolescent athletes with an estimated 2.5 million sports-related injuries presenting to emergency departments (“EDs”) annually.21 Additionally, there are more than one million joint replacements per year in the U.S. with over 790,000 knee replacements done by physicians.
The differential diagnosis of nonspecific musculoskeletal complaints is challenging, and the use of imaging modalities is often required to establish a diagnosis, guide treatment, or monitor disease progression. MRI is a widely used medical technology and is often employed as the preferred imaging tool for disorders of the musculoskeletal system, as it can better delineate soft tissue structures than either plain X rays or CT despite being costlier and having a longer procedural time
20 See, JAMA, Lost Productive Time and Cost Due to Common Pain Conditions in the U.S. Workforce (Nov. 12, 2003), available at https://jamanetwork.com/journals/jama/fullarticle/197628.
21 See, American College of Rheumatology, Joint Replacement Surgery, available at https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Treatments/Joint-Replacement-Surgery#:~:text=Approximately%20790%2C000%20total%20knee%20replacements,in%20any%20area %20of%20medicine (last visited Feb. 10, 2023).
19
compared with CT. Currently there is no optimal imaging technology for imaging implanted orthopedic prosthetic devices. MRI and CT scanners produce confounding artifacts that make these devices less than satisfactory for this application. As previously discussed, CT employs ionizing radiation and MRI frequently requires heavy-metal injection. In addition, the closed environment of an MRI is challenging for many patients and intolerable for others.
Ultrasonography is a noninvasive imaging modality used for the assessment of the musculoskeletal system. It can provide clinically useful information on a wide range of pathologic conditions affecting components of the knee joint, including the tendons, ligaments, muscles, synovial space, articular cartilage, and surrounding soft tissues. Color and power Doppler techniques can be used to measure neovascularization within the synovial lining of the joints, tendons, and soft-tissue masses. The advantages of ultrasound include low cost, portability, real-time assessment, no radiation and facilitated side-by-side comparisons. Its major disadvantage is its operator-dependence: it requires trained experienced hands with appropriate high-resolution equipment. Ultrasound examinations of the knee joint are usually performed using a high-frequency linear transducer (7.5–12 MHz). It is mostly used to diagnose tendon, ligament or muscle injury and cartilage and meniscal lesions.
The Proposed Products—QT Imaging Platform for extremity, infant, and whole-body imaging
The proposed QT Orthopedic Scanner for Extremity Imaging (Image 11 and Image 12) will use the open, partial angle configuration with the same platform technology as the QT Breast Scanner. Using transmission and reflection ultrasound, the system generates high definition (sub-millimeter) extremity images that provide unique visual information about the physical structures within the human musculoskeletal system. The new image information is expected to provide a safe (no radiation or injection), effective, inexpensive, and non-invasive diagnostic imaging tool for assessing musculoskeletal health. With the QT Orthopedic Scanner, the patient sits comfortably on a chair in front of the scanner that contains an opening through which the arm or leg is placed. The extremity is gently immobilized using an inflatable rubber cuff. On the other side of the rubber cuff there is a warm water bath with an ultrasound armature that rotates 325 degrees around the extremity to produce 3D images. The QT Orthopedic Scanner will differ from conventional ultrasound in that it will utilize reflection and transmission data from sound waves, providing a significant increase in diagnostic information using the speed of sound characteristics of the bones and muscles and any prosthetic devices and generating a true 3D rendering of the extremity. The QT Orthopedic Scanner will provide sub-millimeter image resolution called a QTscan which will enable identification of normal and abnormal structures and the accurate depiction of the precise shape and location of findings.

The proposed QT Orthopedic Scanner being applied to hip imaging
Image 11
20

The proposed QT Orthopedic Scanner being applied to shoulder imaging
Image 12
21
Image reconstruction of the ultrasound data is done with proprietary partial-angle segmentation software that uses the quantitative speed of sound data to highlight specific tissues for 3D visualization called a QTscan as shown in Image 13.

Volume reconstructions of the knee from QTScan data
Image 13
22
Clinical Images
In response to a request by the FDA to include Visual Grading Analysis studies in our applications, the Company has conducted an analysis comparing the image quality of the QTScan to MRI in which readers independently scored the image quality of 10 anatomical knee structures with MRI and the QTscan. In this Visual Grading Analysis, readers scored the transmission ultrasound images as equivalent or better than the MRI imaging in more than 90% of knees structure images reviewed. Image 14 below shows the QTScan of the knee (left) next to the MRI imaging of the knee (right). Note the higher contrast in the QTscan compared to the MRI. This exercise was conducted as part of our FDA application process and has not been published.
 |  | |||||||
QTScan (left) and MRI imaging (right) of a human knee
Image 14
The following, Image 15 shows QTScan of the same knee from different views. Note the high contrast and detail in the QTScan image.

Image 15
23
Image 16 below shows QTScan of a human knee (left) compared with MRI views of the same knee (right). Note the higher contrast and detail in the QT image.

QTScan (left) and MRI imaging (right) of a human knee
Image 16
Image 17 below is another set of QTScan views of the same knee. Note the meniscus and cartilage detail in the QTscan images, which cannot be seen on an MRI.

QTscan of a human knee
Image 17
24
The Company has conducted partial-angle reconstruction studies comparing the image quality of 325-degree rotation (open angle) to 360-degree (full rotation) reconstructions (Image 18). The image quality is maintained in the partial-angle reconstruction.

360-degree rotation view (left) vs partial angle reconstruction (right) of the human knee
Image 18
Advantages of proposed QT Orthopedic Scanner
Compared to existing orthopedic imaging systems such as MRI and CT, the proposed QT Orthopedic Scanner will offer the following advantages:
•The QT Orthopedic Scanner may be faster at image acquisition, resulting in quicker diagnosis and treatment.
•In-office and same day orthopedic imaging
•The QT Orthopedic Scanner would not require build-out of a dedicated facility with magnetic field shielding and liquid helium supply (where needed).
•The QT Orthopedic Scanner may be less expensive to deploy than an MRI or CT device and may be less costly to maintain because the technology is simpler in design, has less components, does not utilize ionizing radiation, helium, and we anticipate will be less expensive to manufacture and maintain.
Proposed QT Infant Scanner—Whole Body Imaging
The Need: Currently there are very limited techniques for imaging infants. The QT Infant Scanner is in the development phase.
Background:
Medical imaging is an extremely valuable tool in diagnosing infants and children but poses specific challenges that the proposed QT Infant Scanner would address. At present neonatal and pediatric imaging is severely limited as described below.
CT uses ionizing radiation, which poses greater risk for the pediatric than adult population. The risk associated with ionizing radiation is “higher than in adults. Also, children have longer life expectancy; therefore, they have a greater
25
potential for manifestation of possible harmful effects of radiation.”22 Ionizing radiation exposure in childhood in particular raises risk of cancer, including leukemia, breast cancer, thyroid cancer and brain cancer, where higher risk is associated with exposure any time before age two23. This makes MRI preferred to CT for all but trauma evaluation.
MRI requires sedation or general anesthesia and may require injection of a heavy-metal contrast agent. The use of sedation or anesthetic drugs risks severe compromise of respiratory and cardiac function and injection of contrast is usually contraindicated in seriously ill children due to the high risk of organ failure from the administration of these contrast agents.
Finally, pediatric patients are particularly sensitive to environment given an infant or child’s inability to fully comprehend the nature and purpose of medical imaging. The presence of a parent or caregiver can increase imaging efficacy, but is limited in a closed environment (e.g., MRI) or an unsafe environment (e.g., any modality using ionizing radiation).
The proposed QT Infant Scanner will address all three of these issues as it will not require ionizing radiation or anesthesia and the open environment would allow a trusted adult to be present, decreasing the necessity of sedation and increasing imaging efficacy.
Although prior literature from the Company may have indicated a specific timeframe for proof of concept and rollout, there is currently no specific timeframe for the submission of premarket notification to the FDA for approval of the QT Infant Scanner. Our submission to the FDA for all products and product candidates may depend upon a number of factors and variables, including the amount of capital that we have; the completion of the clinical prototype scanner; the results from the initial pre-clinical imaging studies and comparisons with MRI; the development of an FDA-strategy-for-submission, including but not limited to pre-sub-meetings with the FDA; determining the appropriate device classification and whether it meets criteria for a 510(k) pathway, and final preparation of the FDA application.
22 See, National Library of Medicine, Problems and Preferences in Pediatric Imaging (Oct. 2015), available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4693383/.
23 WHO, Ionizing Radiation, Health Effects and Protective Measures (Apr. 29, 2016), available at https://www.who.int/news-room/fact-sheets/detail/ionizing-radiation-health-effects-and-protective-measures.
26
The Proposed Product—QT Infant Scanner
The imaging from the proposed QT Infant Scanner (the “Open Partial Angle Scanner”) Imaging (Image 19) will be based on the same platform transmission ultrasound technology as the breast and extremity scanners, and uses the Company’s Open Partial Angle Scanner concept.

The Open Partial Angle Scanner will be applied to infant body imaging in the proposed QT Infant Scanner (concept drawing)
Image 19
27
Clinical Images
The Company has not imaged infants but has demonstrated its ability to image the body through imaging of neonatal pigs. Comparative images for QTscan vs MRI are shown below for a neonatal pig (Image 20). Note the higher contrast and more detail in the QTscan (left).
 | ||||||||
| Whole Body QTscan | Whole Body MRI Scan | |||||||
Newborn piglet whole body imaging using the Company’s technology.
Image 20
Other anatomic detail in the newborn pig’s heart and lungs are shown in Images 21, 22, 23 and 24 using the Company’s technology.
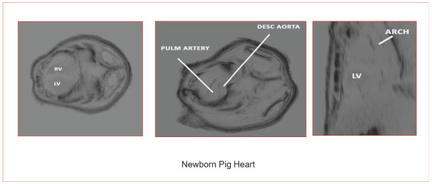
Image 21 (left), Image 22 (center) and Image 23 (right)
28

Piglet Lung Imaging
Image 24
The Company has done partial-angle image reconstruction internal studies comparing the image quality of 325-degree rotation (open angle) to 360-degree (full rotation) reconstructions of a piglet’s pelvis (Image 25). We believe the image quality is maintained in the partial-angle reconstruction.

All views (left) vs partial angle reconstruction (right) of the piglet pelvis
Image 25
The Company’s Image Guided Procedures
The Need: In addition to using the Open Partial Angle Scanner as the proposed QT Infant Scanner, it can also be used for a variety of other image guided procedures including:
•Breast biopsy of small lesions (<5mm)
•Orthopedic biopsy of bones, joints, muscle or connective tissues
29
•Orthopedic injections
•Stem cell injections
•Soft tissue ablation
•Real-time non-radiation imaging of vascular procedures
•Angiography without radiation
•Cryoablation for early-stage breast cancer.
Cryoablation—An example of the Company’s potential contribution
Cryoablation24 is currently approved for treatment of benign and malignant soft tissue tumors by the FDA. Currently, there are no specific technologies that have FDA approval for breast tumors, although there are over 100,000 such procedures done in the U.S. annually.25,26 Eighty-five percent of breast cancer is localized at the time of diagnosis (62% have early stage confined to the breast and 23% have pre-cancerous In Situ carcinoma).27
Cryoablation for cancer is typically used when surgery isn’t an option. Cryoablation is sometimes used as a treatment for many types of cancer, including:
•Bone cancer.
•Breast cancer.
•Cervical cancer.
•Eye cancer.
•Kidney cancer.
•Liver cancer.
•Lung cancer.
•Prostate cancer.
Mastectomy has no advantage over local removal of breast cancer in terms of survival28 and the trend is towards less invasive or disfiguring treatments for treating the primary tumor in the breast. Cryoablation is an emerging modality of treatment with a number of different indications for use.29 Systems such as the Galil Cryoablation System30 are used in clinical practice for a variety of applications. In one study cancer recurred in only one of 180 women treated with cryoablation for low-risk breast cancers.31 There are a number of ongoing trials in this area32, and cryoablation is offered for low-risk breast cancers at more than 20 tertiary cancer centers in the U.S.33
24 Cryoablation is a process that uses extreme cold to destroy abnormal tissue. According to the Mayo Clinic: During cryoablation, a thin, wandlike needle called a cryoprobe is inserted through the skin. The cryoprobe is placed directly into the cancer. A gas is pumped into cryoprobe to freeze the tissue. Then the tissue is allowed to thaw. The freezing and thawing process is repeated several times
25 See, National Library of Medicine, Office-Based Cryoablation of Breast Fibroadenomas with Long-term Follow-up (Sept. 15, 2005), available at https://pubmed.ncbi.nlm.nih.gov/16174156/.
26 See, the American Society of Breast Surgeons, Consensus Guideline on the Use of Transcutaneous and Percutaneous Ablation for the Treatment of Benign and Malignant Tumors of the Breast (Oct. 16, 2018), available at https://www.breastsurgeons.org/docs/statements/Consensus-Guideline-on-the-Use-of-Transcutaneous-and-Percutaneous-Methods-for-the-Treatment-of-Benign-and-Malignant-Tumors-of-the-Breast.pdf.
27 See, ASCO, Breast Cancer: Statistics (Jan. 2022), available at https://www.cancer.net/cancer-types/breast-cancer/statistics.
28 See, JAMA Network, Use of and Mortality After Bilateral Mastectomy Compared with Other Surgical Treatments for Breast Cancer in California, 1998-2011 (Sept. 3, 2014), available at https://jamanetwork.com/journals/jama/fullarticle/1900512.
29 See, DovePress, Cryoablation in the Management of Breast Cancer: Evidence to Date (July 23, 2019), available at https://www.dovepress.com/cryoablation-in-the-management-of-breast-cancer-evidence-to-date-peer-reviewed-fulltext-article-BCTT.
30 See, Boston Scientific, Cryoablation available at https://www.bostonscientific.com/en-US/products/cryoablation.html.
31 InterventionalNews, Cryoablation Shows Promise in Treating Low-Risk Breast Cancer (Jan. 8, 2019), available at https://interventionalnews.com/cryoablation-breast-cancers/.
32 U.S. National Library of Medicine, Cryoablation Therapy in Treating Patients with Invasive Ductal Breast Cancer, available at https://clinicaltrials.gov/ct2/show/NCT00723294 (last visited Feb. 10, 2023).
33 Healio, Cryoablation May be Promising Alternative to Surgery for Low-Risk Breast Cancer (Mar. 4, 2019), available at https://www.healio.com/hematology-oncology/breast-cancer/news/online/%7Be2c51338-c13b-44f6-8690-b01307340d21%7D/cryoablation-may-be-promising-alternative-to-surgery-for-low-risk-breast-cancer.
30
Cryoablation of early-stage breast cancer is an exciting opportunity unique to the Company. Breast cancer cells are about 20 microns wide. A 1-cm cancer has about 100 million cells, a 0.5-cm cancer has about 10 million cells, and a 1-mm cancer has about 100 thousand cells.34 The Company’s imaging can see the glandular structure of the breast and can see as few as a couple of thousand cells. These cancers of several hundred thousand cells are very low risk to the woman and are also easily eliminated35. There are currently limited ways to treat these small cancers using image guided procedures. The Company could offer a solution to this problem.
The Product
The product for image-guided procedures would be the Open Partial Angle Scanner augmented with enhanced software. The Open Breast Scanner is shown in Image 26 below (concept drawing).

The Open Partial Angle Scanner for Breast Imaging
Image 26
34 National Library of Medicine, Disappearing Breast Cancers (Apr. 2012), available at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3320224/.
35 See, AJR, Robert C. Ward, Ana P. Lourenco & Martha B. Mainiero, Ultrasound-Guided Breast Cancer Cryoablation, 213 Am. J. Roentgenol. 3, 716-722 (2019), available at https://www.ajronline.org/doi/10.2214/AJR.19.21329.
31
The Open Partial Angle Scanner or Open Breast Scanner will be able to operate with the same accuracy as the full 360º rotation all views breast scan, as shown below (Image 27):

All views breast reconstruction (left) vs open angle breast reconstruction (right) of the same human breast
Image 27
Furthermore, the images reconstructed of scans performed on the open-angle scanner do not show any significant artifacts in the presence of an intervening device (e.g., a needle) (Image 28).
Top view (left panel) and frontal view (right panel)
An ablation needle shown in a human cadaver breast using the Company’s platform
Image 28
Manufacturing
The Company’s products are manufactured in small scale in Novato, California. With the entry into the Canon Manufacturing Agreement as discussed below, the Company will be manufacturing the QT Brast Scanners in parallel between Novato, California and using Canon Medical Systems Corporation (“CMSC”) as a contract manufacturer pursuant to the Canon Medical Agreement Starting with the second half of 2025. The products are designed under the FDA’s design control guidelines and manufactured in accordance with the Company’s quality management system.
The Company’s devices are made up of custom designed components and off-the-shelf components, both of each are supplied by the Company’s approved vendors in the U.S.
32
Currently all subassemblies are manufactured at the Company and verified prior to the final assembly of the device. The controls software and image reconstruction software are loaded on the imaging devices at the Company’s facility in Novato. Prior to shipping, 100% of the products are verified for functionality, performance, and safety.
The Company intends to scale up its production by initially using CMSC, and may also look at strategic original equipment manufacturing (“OEM”) agreements for large production throughput.
The suppliers that the Company purchases from and engages with are limited to those that are approved by our Quality Assurance department, which maintains an Approved Supplier List. The Company categorizes suppliers into three groups: (i) non-critical, (ii) important, and (iii) critical. For example, our “non-critical” suppliers include general distributors and/or suppliers of commercially available “off-the-shelf” items such as mechanical and electrical standard hardware, blank label stock, seals and labeling pouches, and our “important” suppliers include custom component suppliers, test facility providers and consultants.
Before a supplier is classified as “critical,” the Company assesses the supplier’s: (a) specific or proprietary core competencies, (b) tooling costs and lead time, and (c) product delivery lead time. Any supplier whose processes or products are required by the Company to be validated are classified as “critical.” Another factor that the Company considers is the lead time to approve an alternative supplier. As of the date of this annual report, although there are various suppliers in the U.S. and abroad that can produce high quality ultrasound transducers for the Company, the Company has only engaged in the supplier validation and approval process with one such supplier which manufactures ultrasound transducer subcomponents in accordance with the Company’s specifications. Because the Company has validated this supplier, but has not undertaken the significant commitment of resources to validate and approve other such suppliers of ultrasound transducers, despite the fact that the Company could choose to do so, it considers this supplier as being “critical” on our Approved Supplier List but not a principal supplier. In addition, as of the date of this annual report, the Company does not have written agreements in place with this critical supplier, and is operating under an individual purchase order platform, on an as-needed-basis; however, the Company may enter into such agreements in the future.
Canon Letter of Intent
QT Imaging entered into the Canon Letter of Intent with Canon Medical USA, Inc. (“CMSU”) and CMSC pursuant to which CMSC purchased and acquired two QT Breast Scanners in the first half of 2024. The Canon Letter of Intent provided that CMSC would conduct, and pursuant to the Feasibility Study Agreement (as described below), CMSC conducted, feasibility studies on the QT Breast Scanners that it acquired, including product quality validation, development and manufacturing studies, clinical evaluation, regulatory investigation and marketing validation (the “Feasibility Study”) The Feasibility Study was completed in the second half of 2024.
The Canon Letter of Intent provided that upon successful conclusion of the Feasibility Study, we and CMSC intended to engage in a good faith discussion to develop a binding OEM manufacturing agreement with CMSC, which we have now done, as discussed below.
CMSC will also use QT Breast Scanners that it acquired to perform clinical trials towards the possibility of it pursuing the regulatory approval process in Japan.
CMSC and QT Imaging have also discussed other potential terms between them.
Feasibility Agreement with Canon Medical Systems Corporation
On March 28, 2024, the Company entered into the Feasibility Study Agreement with CMSC. The term of the Feasibility Study Agreement commenced on March 28, 2024 and remained in force until the end of December 2024. In connection with the Feasibility Study Agreement, CMSC initiated studies to evaluate the business, technical, and clinical values of the QT Breast Scanner including the Feasibility Study. CMSC has no right to reverse engineer the QT Breast Scanner and may only modify and disassemble the QT Breast Scanner as necessary to conduct the Feasibility Study.
Under the terms of the Feasibility Study Agreement, QT Imaging provided support for the Feasibility Study as agreed with CMSC from time to time during the term of the Feasibility Study Agreement and used its commercially reasonable efforts to facilitate the Feasibility Study.
All know-how and intellectual property embodied in QT Breast Scanner are owned by QT Imaging and all rights not expressly granted to QT Imaging are reserved.
33
Canon Manufacturing Agreement
On March 28, 2025, the Company entered into a manufacturing agreement (the “Canon Manufacturing Agreement”) with CMSC. Pursuant to the terms of the Canon Manufacturing Agreement, the Company appoints CMSC as the exclusive manufacture of the QT Breast Scanners to be distributed by NXC Imaging, a wholly-owned subsidiary of CMSU (“NXC”) pursuant to the Amended Distribution Agreement (as defined below). CMSC’s manufacturing of the QT Breast Scanners shall not include the final testing process for the QT Breast Scanners, and CMSC shall not have any responsibility for the QT Breast Scanner as a manufacturer of medical equipment for FDA purposes.
In order for CMSC to manufacture the QT Breast Scanners, the Company shall sell components to CMSC in a timely manner, in accordance with agreed-upon terms and conditions. The Company shall cooperate with and support CMSC to allow CMSC to procure components through its own supply chain. The parties shall negotiate in good faith specific procedures to phase out CMSC’s purchase of components from the Company, with the target date of the fourth quarter of 2025, although this is not a commitment. The terms for purchasing components (or subassemblies), including components for service, by the Company from CMSC shall be mutually agreed upon by the parties.
The Company shall provide forecasts (each, a “Forecast”) to CMSC in accordance with the “Forecast Policy” attached to the Canon Manufacturing Agreement. No later than forty-five days prior to the end of each calendar quarter, CMSC will notify the Company in writing if CMSC is unable to deliver a sufficient number of QT Breast Scanners to satisfy the Forecast for the following quarter. If CMSC is unable to deliver a sufficient number of QT Breast Scanners due to CMSC’s willful misconduct, CMSC will sell back to the Company all components necessary for the Company to engage in the manufacture of the QT Breast Scanners for the following quarter at the Company’s facility in Novato, California (hereinafter referred to as “Novato Manufacturing”) sufficient to cover the QT Breast Scanner shortfall back at the same purchase price that CMSC paid to purchase such components. CMSC shall bear risk of loss, all fees and shipping costs for the components incurred in connection with such Novato Manufacturing.
No later than forty days prior to the end of each calendar quarter, the Company shall notify CMSC in writing indicating whether or not the Company wishes to engage in Novato Manufacturing of the QT Breast Scanners for the following quarter for the purpose of meeting the Forecast for such quarter. If the Company provides such notice to CMSC within the 40-day period, stating that the Company wishes to engage in Novato Manufacturing, the Company may purchase from CMSC (if the Company does not already have) any components held by CMSC necessary as determined by the Company for the Company to be able to manufacture the QT Breast Scanners via the Novato Manufacturing, at the same purchase price that CMSC paid to the Company to purchase such components. The Company shall bear all risk of loss, fees and shipping costs for the components incurred in connection with the Novato Manufacturing.
From time to time during the term of the Canon Manufacturing Agreement, but no later than thirty days prior to the delivery date specified in the purchase order, the Company may issue to CMSC a purchase order for any of the QT Breast Scanners necessary to meet the Forecast, for the actual quantities to be delivered by CMSC. Each purchase order shall specify delivery dates that respect the “Forecast Policy.” The purchase prices applicable to the purchase orders as of the date of the Canon Manufacturing Agreement shall be separately agreed between the parties in writing. CMSC may reject any purchase order that requests delivery dates that are not in compliance with such Forecast Policy. Unless the Company is manufacturing QT Breast Scanners as Novato Manufacturing, any failure by CMSC, as a result of CMSC’s conduct, to manufacture and deliver QT Breast Scanners as specified in an accepted purchase order necessary to satisfy the Forecasts later than seventy-five days from the delivery date as specified in the accepted purchase order shall result in liquidated damages per missed or delayed QT Breast Scanner of $220,000, with NXC not being obligated to pay for the missed MOQ sale. Such failure by CMSC shall also not provide any grounds for NXC to terminate the Amended Distribution Agreement or otherwise cancel the binding nature of the MOQs (as defined below) as provided in the Amended Distribution Agreement (as described below). All title to and risk of loss of the QT Breast Scanners shall pass from CMSC to the Company when the QT Breast Scanners have been delivered to the Company in Novato, California, under the Incoterms 2020 trade term, DDP. The Company shall make payment of the QT Breast Scanners to CMSC within ninety days after the issuing date of an original copy of the invoice to be issued by CMSC.
Prior to the delivery of the QT Breast Scanners, CMSC shall inspect and test all the QT Breast Scanners in accordance with the outgoing test procedures to be approved in writing by the Company and as specified in the specifications or otherwise to be separately agreed upon between the parties in writing. The Company shall perform the visual, quantity and/or quality inspection of the QT Breast Scanners upon their receipt. If any of the QT Breast Scanners delivered is found deficient in quantity (as set forth in the accepted purchase order), or is defective in material and workmanship or inconformity with the specifications, and such deficiency, defect or non-conformity is caused by CMSC, the Company may submit a written claim notice to CMSC with sufficient supporting evidence, including but not limited to photographs and reasonably detailed explanation. CMSC shall, at its sole discretion, either (i) deliver additional QT Breast Scanners to
34
fulfil the required quantity or deliver replacement of the QT Breast Scanners, or (ii) offer an equitable reduction in the purchase price of the QT Breast Scanners delivered, subject to mutual agreement between the parties. CMSC is providing a warranty on the QT Breast Scanners that they shall be free from defects in material and workmanship and conform to the specifications for a period of (i) twelve months from the delivery date of each QT Breast Scanner from the Company to NXC, or (ii) twelve months from the installation date for the QT Breast Scanners directly sold to end users by the Company. If any defects in material and workmanship, or non-conformity of the specifications occur during the warranty period and the Company provides a timely claim, CMSC shall, at its sole discretion (i) send a replacement of a part(s), (ii) send a brand new QT Breast Scanner as a replacement, (iii) repair the defective QT Breast Scanner(s) and return them to the Company, or (iv) refund the equivalent price to the purchase price. CMSC shall only be responsible for recalls resulting from the manufacturing process of the QT Breast Scanners or materials and parts procured by CMSC (other than materials and parts procured from the Company).
All intellectual property rights, including but not limited to patents, copyrights, know‑how, and trade secrets (the “IPR”), which each party has owned before entry into the Canon Manufacturing Agreement or develops separately from the performance of the manufacture of the QT Breast Scanners under this agreement, shall remain the exclusive property of the Company or CMSC respectively. All IPR in and to any invention, whether patentable or not, conceived and reduced to practice in the performance of the manufacture of the QT Breast Scanners under the Canon Manufacturing Agreement shall be owned solely by CMSC; provided, that to the extent that such invention is based on the Company’s confidential information and is exercised on the QT Breast Scanners which are manufactured by CMSC and delivered to the Company, such as manufacturing process flow, CMSC agrees not to assert any IPR in and to such invention against the Company, its affiliates and customers as to the QT Breast Scanners. All IPR to and in any invention, whether patentable or not, if agreed between the parties to have been conceived and reduced to practice jointly by the Company and CMSC in the performance of the manufacture of the QT Breast Scanners shall be owned jointly and equally by the Company and CMSC, and both parties shall have the right to use, utilize and exploit such IPR in accordance with applicable laws involved without a prior consent of or any payment or consideration to the other party.
The Company hereby grants to CMSC a personal and exclusive license to use, utilize (including copy, amend, revise and modify) and exercise the IPR owned by the Company solely for enabling it to manufacture the QT Breast Scanners sold to NXC and provide maintenance support for them, provided that (i) such exclusive license may be sublicensed to NXC and other subsidiaries of CMSC and/or authorized maintenance companies (with such authorization to be provided in writing by the Company, but not to be unreasonably refused, withheld, or delayed) that provide maintenance services on an outsourced basis (the “Approved Parties”) solely for the QT Breast Scanners distributed by NXC pursuant to the Amended Distribution Agreement, whether or not the QT Breast Scanners are distributed before, on, or after the entry into the Canon Manufacturing Agreement; otherwise such license shall be non-exclusive, non‑sublicensable and non-transferable, and (ii) CMSC shall refrain from using its license to amend, revise and modify the QT Breast Scanners, including the source code(s) of software (which shall be held in escrow pursuant to the terms of a separate escrow agreement among the parties and an escrow agent (the “Escrow Agreement”)), unless the Company becomes party to an event of any bankruptcy or other insolvency proceeding (a “QTI Bankruptcy”).
If there is a QTI Bankruptcy, CMSC may use its license in accordance with the following: in the event of a QTI Bankruptcy and rejection or termination of the Canon Manufacturing Agreement in a QTI Bankruptcy, all source code(s) will be released by the escrow agent to CMSC and CMSC may exercise its rights to amend, revise and modify such source code(s) (A) to enable CMSC to manufacture (x) up to 100 QT Breast Scanners to be sold to NXC or (y) in the event that 100 QT Breast Scanners have yet to be sold to NXC by December 31, 2026, QT Breast Scanners until December 31, 2026, whichever occurs earlier, and (B) to enable CMSC and the Approved Parties until the earlier of (i) the end of the five-year lifetime of the QT Breast Scanners sold to NXC pursuant to the Amended Distribution Agreement and (ii) December 31, 2031 (such earlier date, the “Maintenance Term”) to support the continuous workable operation, but not for upgrades or improvement of performance, of QT Breast Scanners that were distributed by NXC before, on, or after the Canon Manufacturing Agreement. Following the expiration of the Maintenance Term, CMSC shall return to the Company or its successor physical material released to CMSC by the escrow agent and use commercially reasonable efforts to erase electronically submitted material released to it by the escrow agent, provided, however, that CMSC shall be permitted to retain information stored on backup or archival media pursuant to bona fide security measures or for legal compliance. In the event that prior to a QTI Bankruptcy, CMSC terminates the Canon Manufacturing Agreement, other than because the Company has substantially failed to comply with any provision of the Canon Manufacturing Agreement and that such non-compliance has not been cured within thirty business days from the date of the Company’s receipt of the written notice from CMSC, in the event of a QTI Bankruptcy there shall be no release of source code(s). Furthermore, in the event that prior to a QTI Bankruptcy, CMSC terminates this agreement because the Company has substantially failed to comply with any provision of the Canon Manufacturing Agreement and that such non-compliance has not been cured within thirty business days from the date of the Company’s receipt of the written notice from CMSC and there is a subsequent QTI
35
Bankruptcy, the usage by CMSC of the source code released to CMSC pursuant to this Article 9.3.2 shall be limited to subpart (B) set forth in the first sentence of this paragraph. Furthermore, if the Company terminates the Canon Manufacturing Agreement in the event that CMSC substantially fails to comply with any provision of the Canon Manufacturing Agreement and that such non‑compliance has not been cured within thirty business days from the date of its receipt of the written notice from the Company, then there shall be no release of source code(s) to CMSC.
Unless earlier terminated in accordance with the terms of the Canon Manufacturing Agreement, this agreement shall remain effective until December 31, 2026. In case both parties mutually agree the extension of the Canon Manufacturing Agreement in writing, the Canon Manufacturing Agreement shall be extended for one year after the expiration of the Canon Manufacturing Agreement. In the event of “Change of Control” of a party, either party shall have the right to, at its sole discretion, terminate the Canon Manufacturing Agreement in its entirety upon forty-five business days’ prior written notice to the other party. Such notice shall be provided prior to the expiration of the then current term of the Canon Manufacturing Agreement. In the event of the termination or expiration of the Canon Manufacturing Agreement, CMSC will honor all of the outstanding accepted purchase orders, and both parties will cooperate to fulfill such orders. In the event that either party substantially fails to comply with any provision of the Canon Manufacturing Agreement and that such non-compliance has not been cured within thirty business days from the date of its receipt of the written notice from the other party, then the other party shall have the right to terminate the Canon Manufacturing Agreement upon giving a written notice. The Canon Manufacturing Agreement also provides other grounds for its termination prior to December 31, 2026, including by CMSC if NXC’s sales of the QT Breast Scanner are adversely affected due to any failures or other circumstances attributable to the Company that the Company is or is reasonably expected to be unable to remedy (including but not limited to Epidemic Failures; the submission of Medical Device Reports (MDRs) to the FDA regarding the QT Breast Scanner; any action by the Company (either voluntarily or upon an order or request by the FDA) to conduct a Field Correction, Market Withdrawal, Stock Recovery, or Recall (as such terms are defined by the FDA); any action by the Company or any regulatory authority to temporarily suspend shipments or manufacturing of QT Breast Scanners; or issues related to the Company’s business operations) within six months from the date of the onset of such an event. For these purposes, “Epidemic Failures” means a malfunctioning of five or more QT Breast Scanners collectively presumed to have a common underlying cause based on a reasonable evaluation. Following a termination or expiration of the Canon Manufacturing Agreement, the provisions with respect to the grants of licenses to the IPR of the Company will survive up to the earlier of (a) the end of the five-year lifetime of the QT Breast Scanners sold to NXC and (b) December 31, 2031.
The foregoing description is qualified in its entirety by reference to the Canon Manufacturing Agreement, a copy of which is attached hereto as Exhibit 10.46 and incorporated herein by reference.
Sales and Marketing
The Company’s primary sales and marketing efforts in the short-to-medium term will be to focus on the $3 billion breast imaging market in United States supported by the exclusive partnership with our distribution partner, NXC Imaging.
Leverage Current Clearances to Build Presence and Awareness in the Medical Community
The current QT Breast Scanner is a Class II device subject to premarket notification and clearance under Section 510(k) of the FDCA. The QT Breast Scanner is currently cleared by the FDA under Section 510(k) (which clearance was granted in June 2017) for breast imaging but not as a replacement for screening mammography – currently, the device has FDA approval and can be reimbursed in cases where additional breast imaging is necessary. This includes women who need adjunctive screening, such as: women with dense breasts or where there is a finding; high risk women below the recommended age for mammography; and women who would benefit from more frequent breast imaging, such as women undergoing treatment or women on prophylactic medication to prevent breast cancer.
Many patients may find QT Imaging modality preferable for dense breasts, implants, post therapy screening where breasts can be very sensitive to compression and where patients have concerns about the radiation dose.
A particular opportunity for the Company under its current clearance is to provide a backup option for women who are recommended for a breast MRI but are unwilling or unable to have it for cost or accessibility reasons. With additional clinical data comparing the clinical efficacy of the QT Breast Scanner to MRIs, the QT Breast Scanner may become a less expensive, more patient-friendly alternative to MRIs (no contrast-dye, no claustrophobia, no noise). QT Scanners will free MRI scanners for other non-breast imaging studies.
Achieving clinical adoption requires building awareness and acceptance in the medical community. The first step will be to place machines with early adopters who see the benefits of the Company’s technology, are interested in using the
36
device in their practice, are willing to collect data on its use, and will publish results or speak to their peers about its clinical value.
An example of this strategy in practice is the National Cancer Institute grant received in 2022 in partnership with the University of Illinois – Urbana-Champaign whereby the Company will place a QT Breast Scanner at St. Margaret’s Hospital in Toronto, Canada. The objective is to evaluate and measure the effectiveness of the QTscan in evaluation and monitoring cancer treatment in comparison to breast MRI which is currently the standard-of-care in quantitative monitoring of response to therapy. The results of the study will be published and would represent an independent validation of the clinical value of the QTscan with the imprimatur of a respected university, hospital, and the National Cancer Institute. The Company intends to establish similar peer-review partnerships with respected medical organizations, practices, and practitioners in the future.
Sales and Marketing Strategy
The Company’s sales efforts are supported by partnership with strategic sales and distributors partners, with solid channels in the industry and large sales organizations. The Company will scale scanner placements in hospitals, imaging centers, and health centers via such partnerships.
Use an Installed Base as a Platform to Expand Awareness and Produce Additional Data for Clinical Acceptance, Reimbursement, and Additional FDA Clearances
Part of the challenge of achieving clinical acceptance and adoption of the Company’s technology is data that proves the efficacy of the QTscan relative to the current standard of care for breast imaging in screening, diagnosis and treatment. The rollout of QT Breast Scanners as described in the previous section will provide multiple means of collecting this data for clinicians, which can also be used to support reimbursement and expanded FDA clearances of the QT Breast Scanner as an alternative to screening breast MRI in younger women (under 40 years of age) with above average risk of breast cancer.
Data collection and analysis, support reimbursement and expanding FDA clearances are time-consuming, but with the help of the installations that will be accelerated through the placement programs detailed above, the Company believes that it can achieve these objectives with maximum efficiency.
Market Segments
As the installed base for QT Breast Scanners expands, and as discussed above, the Company intends to tailor its marketing efforts towards three segments as it builds awareness and acceptance for its imaging technology. All are important for success, and each requires its own strategy and messaging.
•Patients. This is the end-user/consumer – the women who are dissatisfied with the current scanning model. The Company intends to approach patients through multiple channels, beginning with outreach efforts to recruit key influencers and opinion leaders. These individuals would be recruited through networking and education via targeted interest groups and would become advocates of the benefits of the Company’s technology to their respective groups.
•Medical Professionals/Radiologists. In addition to marketing to women, the Company launched an intensive campaign aimed at the medical community. This is a more difficult effort than that aimed at women – from the provider perspective, the need for multiple scans and callbacks that are a negative for patients are actually seen as an economic positive as additional patient visits generate a significant amount of revenue. This is true for both institutions and radiologists, and neither have a great incentive to deviate from the current status quo.
NXC Distribution Agreement
We are supported by a strong distribution and business partnership with NXC. On May 13, 2023, QT Imaging entered into the NXC Sales Agent Agreement with NXC, pursuant to which QT Imaging appointed NXC as the non-exclusive agent for the sale of the QT Breast Scanner and related services in non-exclusive territories: the U.S., U.S. territories, and U.S. Department of Defense installations. Additionally, NXC was appointed as the exclusive servicer of the QT Breast Scanners sold by NXC under the terms of the NXC Sales Agent Agreement. Under the NXC Sales Agent Agreement, QT Imaging had the right to set the price for its products and agreed to pay NXC a commission based on the purchase order price charged to a customer. Pursuant to the NXC Sales Agent Agreement, NXC was responsible for promotion and sale of the QT Breast Scanner and related services within the designated territory, as well as servicing the QT Breast Scanners sold by NXC. The initial term of the NXC Sales Agent Agreement was for three years.
37
Subsequently, effective June 10, 2024, we and NXC replaced the NXC Sales Agent Agreement with the NXC Distribution Agreement. Under the NXC Distribution Agreement, NXC is appointed as the exclusive reseller to market, advertise, and resell QT Breast Scanners in the U.S. and U.S. territories. NXC will purchase for the purpose of reselling, leasing or renting QT Breast Scanners directly to its customers, but is not obligated to purchase any particular quantity of QT Breast Scanners from us. We have reserved the right to sell directly to customers as an exception. Furthermore, we may, in our sole discretion, sell the QT Breast Scanners to any other person or entity anywhere in the world without notice to NXC or NXC’s prior consent. NXC is also allowed to assign sales agents for the purpose of QT Breast Scanner sales. NXC’s purchases will be in accordance with an agreed upon product pricing schedule (subject to change upon 60 days’ prior written notice by us), provided that neither NXC nor its assigned sales agents may mark-up the cost of the QT Breast Scanners more than twenty percent (20%) unless otherwise mutually agreed to between NXC and us. Each order will include information reasonably requested by us and is subject to our acceptance, after which it becomes an approved order. Any such approved orders are non-cancellable and not subject to rescheduling after acceptance by us. Any orders not accepted by us in writing are deemed rejected.
On October 29, 2024, we and NXC entered into Amendment No. 1 to the Distribution Agreement (the “First Amendment”) to expand Section 20 of the NXC Distribution Agreement to provide that NXC shall be obligated to inform its customers who purchase the QT Breast Scanner (the “Equipment”) that such customers shall not (i) use, copy, distribute, display, perform, or prepare derivative works of any materials accompanying or embodied in the Equipment (except to the extent expressly permitted by such customer’s license to such Equipment and its documentation) or (ii) use any Seller Marks (as defined in the NXC Distribution Agreement), in each case without our prior written consent. Further, the First Amendment provides that upon any unauthorized use of the Seller Marks or materials accompanying the Equipment by any customer, NXC shall promptly inform us and provide all reasonably requested assistance in termination such unauthorized use.
On December 11, 2024, we and NXC entered into the Amended Distribution Agreement, which amends and restates the NXC Distribution Agreement in its entirety. The Amended Distribution Agreement provides for the following modifications to the NXC Distribution Agreement, with the balance of terms (including those added by the First Amendment) remaining materially unchanged:
Sale of Equipment
Under terms of the Amended Distribution Agreement, in the event that CMSC enters into an OEM manufacturing agreement, then fulfillment by the parties to such manufacturing agreement of their respective obligations under such manufacturing agreement shall be a condition to NXC being the exclusive reseller to market, advertise, and resell the Equipment in the U.S. and U.S. territories.
Minimum Order Quantities and Pricing
The Amended Distribution Agreement provides that no later than five days prior to the end of each calendar quarter, NXC shall provide to us a forecast of the anticipated purchases of Equipment during the subsequent twelve-month period. The Amended Distribution Agreement further provides that the forecast for 2025 and 2026 shall be no less than the Minimum Order Quantities (the “MOQs”) set forth in an exhibit to the Amended Distribution Agreement, by quarter and by year. Furthermore, all purchase orders from NXC shall be for no less than the MOQs, which NXC must order on the quarterly and annual basis as set forth in an exhibit to the Amended Distribution Agreement. However, in the event that the we and CMSC do not enter into an OEM manufacturing agreement, then the MOQs shall be non-binding only in the event that we cannot fulfill the manufacture and delivery volumes required for NXC to meet the MOQs.
Should NXC fail to submit a purchase order for no less than the MOQs in any quarterly or annual period, then we may invoice NXC and NXC shall pay us for the difference between the Equipment purchased and the MOQs for such period.
NXC’s purchases will be in accordance with a product pricing schedule attached to the Distribution Agreement as an exhibit (subject to change upon 60 days’ prior written notice by us). The Amended Distribution Agreement removed the cap on markup by NXC on its resale of the cost of the Equipment that was provided for by the NXC Distribution Agreement, such that NXC may set the resale price for customers at its sole discretion.
Payment Terms
Except as otherwise set forth in an applicable Approved Order, the Amended Distribution Agreement provides that we will invoice NXC upon shipment of the Equipment and NXC shall pay the invoice by net thirty days from shipment of the Equipment.
38
After Sale Service
The Amended Distribution Agreement obligates us to continue to provide technical support, spare parts, and necessary know-how in order for NXC to continue to service and support Equipment for at least five years after installation of the Equipment at a customer site. The Amended Distribution Agreement also deleted a provision in the NXC Distribution Agreement that required NXC to request each of its customers to have a qualified or trained breast radiologist.
Limited Warranty
The Amended Distribution Agreement contains limited warranties with respect to the Equipment and relevant spare parts, to remain in effect (a) for Equipment for the shorter of fifteen months from the shipment of the Equipment or twelve months from the date of customer acceptance of installed Equipment, and (b) the relevant spare parts for the shorter of twelve months from the date of their shipment and six months from the date that their completion is installed.
Non-Solicitation
The Amended Distribution Agreement contains a non-solicitation provision stating that we agree, during the term thereof and for a period of three years after, we shall not, directly or indirectly: (a) interfere with or attempt to interfere with any relationship between NXC and any of its distributors, agents, employees, consultants, independent contractors, agents or representatives, (b) solicit the business or accounts of NXC, or (c) divert or attempt to direct from NXC any business or interfere with any relationship between the NXC or any of its clients, suppliers, customers or other business relations; provided, however, that we may engage with the end customers that have acquired the Equipment to the extent necessary to enable such end customers to utilize the Equipment. Furthermore, to the extent not otherwise prohibited by law, each party agrees that, during the term of the Amended Distribution Agreement and for a period of three years after, each party shall not, directly or indirectly solicit for employment the employees of the other except to the extent that such solicitation is done through a general advertisement or solicitation that is not specifically targeting the employees of the other.
Term
The Amended Distribution Agreement extends the term from December 31, 2025 until December 31, 2026, unless earlier terminated or extended by mutual written agreement.
On March 28, 2025, we entered into Amendment No. 1 to the Amended and Restated Distribution Agreement (the “First Amendment to A&R Distribution Agreement”). The First Amendment to A&R Distribution Agreement amends certain provisions of the Amended Distribution Agreement. It provides, among other things, that we reserve the right to sell the Equipment directly to a third party as an exception, and any such direct sale by us shall occur after a prior written notification to NXC and shall count towards the MOQs, provided that if a third party is a competitor of NXC as defined in the First Amendment to A&R Distribution Agreement or an existing customer of NXC or NXC’s reseller or sales agent at the time when we seek to sell the Equipment directly to the third party, we may not sell the Equipment to such third party. It also provides that NXC shall provide us with the forecast of the anticipated purchases of Equipment during the subsequent twelve-month period no later than forty-five days prior to the end of each calendar quarter, rather than five days prior to the end of each calendar quarter. No changes are made to the amount of the MOQs by the First Amendment to A&R Distribution Agreement, but the MOQs shall not be binding if at any time (a) we cannot fulfill the manufacturing and delivery volumes required for NXC to meet the quarterly MOQs, including due to (i) failures or other circumstances attributable to us including, but not limited to, issues relating to design or performance of the Equipment (e.g., failure of the Equipment to meet its published specifications), orders by the FDA or another governmental organization to temporarily or permanently suspend shipments, and to activities or operations of QTI, or (ii) a final non-appealable judgment that would impair our ability to fulfill such manufacturing and distribution volumes as a result of (A) intellectual property infringement made by a third party relating to the Equipment or (B) other legal proceedings against us; or (b) we engage with any competitor or existing customers of NXC or its reseller or sales agent to sell the Equipment in violation of the terms of the Amended Distribution Agreement, as amended. Furthermore, the First Amendment to A&R Distribution Agreement provides that in the event NXC is prevented from selling the Equipment due to actions of or attributable to us, we and NXC will discuss in good faith. We also agreed to introduce at least one new product improvement per year to the Equipment, that we will also timely correct all Equipment bugs or other defects as necessary to address performance issues, and that any increases of the price of the Equipment shall be upon mutual agreement of the parties. Also, in the event of an Equipment Recall (as such term is defined in the First Amendment to A&R Distribution Agreement), the First Amendment to A&R Distribution Agreement provides both parties shall promptly consult and cooperate with the other party, including with respect to the appropriate action to be taken in connection with such Equipment Recall, and mutually agree to
39
appropriately modify the MOQs for the respective quarter or until the Equipment Recall is corrected with mutual agreement with NXC.
The foregoing description is qualified in its entirety by reference to the First Amendment to A&R Distribution Agreement, a copy of which is attached hereto as Exhibit 10.47 and incorporated herein by reference.
Selectively Consider Offshore Marketing Opportunities
Although we have primarily focused our resources on the U.S. market, QT Imaging did commence some marketing initiatives outside the U.S. Pursuant to the Innovador Distribution Agreement between QT Imaging and Innovador, dated November 2, 2022, QT Imaging appointed Innovador as QT Imaging’s distributor for much of Asia. The Asia market is attractive as the incidence of dense breast tissue in Asian women36 is higher than that in the U.S. women37.The territory for the Innovador Distribution Agreement includes Singapore, Malaysia, Thailand, Indonesia, Philippines, Myanmar, Vietnam, Cambodia, Laos, Brunei, India, Pakistan, Sri Lanka, Bangladesh, Nepal, Mongolia, Taiwan, Hong Kong, and Macau. Under the Innovador Distribution Agreement, QT Imaging is responsible for developing and manufacturing its products and supporting Innovador’s product registration and sales and marketing efforts, and Innovador is responsible for product registration, market development, sales & marketing, distribution, and service of the QT Imaging products. Under the Innovador Distribution Agreement, Innovador provides QT Imaging with nonbinding forecasts of the volume of QT Imaging’s products it expects to sell each year. Innovador takes possession of any machines it purchases.
The initial term of the Innovador Distribution Agreement is three years. Either party may terminate the Innovador Distribution Agreement if the counterparty breaches the agreement, engages in fraudulent conduct, becomes insolvent or is adjudicated bankrupt, or fails to function as a viable and operative concern or to conduct its operations in the normal course of business.
Government Regulation
Our existing product, the QT Breast Scanner, products under development, and our operations are subject to extensive regulation by the FDA, and other federal and state authorities in the U.S., as well as comparable authorities in foreign jurisdictions. Our products do not emit radiation, but are subject to regulation as medical devices in the U.S. under the FDCA and as implemented and enforced by the FDA, and under comparable regulatory schemes in foreign jurisdictions.
FDA Regulation of Medical Devices
The FDA regulates the development, design, non-clinical and clinical research, manufacturing, safety, efficacy, labeling, packaging, storage, installation, servicing, recordkeeping, premarket clearance or approval, adverse event reporting, advertising, promotion, marketing and distribution, and import and export of medical devices to ensure that medical devices distributed within the U.S. are safe and effective for their intended uses and otherwise meet the requirements of the FDCA.
FDA Premarket Clearance and Approval Requirements
Subject to certain exceptions, each medical device commercially distributed in the U.S. requires either FDA clearance of a 510(k) premarket notification, or approval of a pre-market approval application (“PMA”). Under the FDCA, medical devices are classified into one of three classes-Class I, Class II or Class III-depending on the degree of risk associated with each medical device and the extent of manufacturer and regulatory control needed to ensure its safety and effectiveness. Class I includes devices with the lowest risk to the patient and are those for which safety and effectiveness can be assured by adherence to the FDA’s General Controls for medical devices, which include compliance with the applicable portions of Quality System Regulation (“QSR”), facility registration and product listing, reporting of adverse medical events and truthful and non-misleading labeling, advertising and promotional materials. Class II devices are subject to the FDA’s General Controls, and special controls as deemed necessary by the FDA to ensure the safety and effectiveness of the device. These special controls can include performance standards, post-market surveillance, patient registries and FDA guidance documents.
While most Class I devices are exempt from the 510(k) premarket notification requirement, manufacturers of most Class II devices are required to submit to the FDA a premarket notification under Section 510(k) of the FDCA requesting permission to commercially distribute the device. The FDA’s permission to commercially distribute a device subject to a
36 See, American Journal of Roentgenology, Mammographic Breast Density and Race (Apr. 2007), Table 1, available at https://www.ajronline.org/doi/10.2214/AJR.06.0619#:~:text=This%20study%20shows%20that%20Asians.
37 See, CDC, What does it Mean to Have Dense Breasts, available at https://www.cdc.gov/breast-cancer/about/dense-breasts.html?CDC_AAref_Val=https://www.cdc.gov/cancer/breast/basic_info/dense-breasts.htm (last visited Jan. 4, 2024).
40
510(k) premarket notification is generally known as 510(k) clearance. Devices deemed by the FDA to pose the greatest risks, such as life sustaining, life supporting or some implantable devices, or devices that have a new intended use, or use advanced technology that is not substantially equivalent to that of a legally marketed device, are placed in Class III, requiring approval of a PMA. Some pre-amendment devices are unclassified, but are subject to the FDA’s premarket notification and clearance process in order to be commercially distributed.
510(k) Clearance Marketing Pathway
The current QT Breast Scanner is a Class II device, and we expect products under development such as the QT Infant Scanner and the QT Orthopedic Scanner will also be Class II devices subject to premarket notification and clearance under section 510(k) of the FDCA. To obtain 510(k) clearance, we must submit to the FDA a premarket notification submission demonstrating that the proposed device is “substantially equivalent” to a predicate device already on the market. A predicate device is a legally marketed device that is not subject to premarket approval, i.e., a device that was legally marketed prior to May 28, 1976 (pre-amendments device), and for which a PMA is not required, a device that has been reclassified from Class III to Class II or I, or a device that was found substantially equivalent through the 510(k) process. The FDA’s 510(k) clearance process usually takes from three to twelve months, but often takes longer. The FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence. In addition, the FDA collects user fees for certain medical device submissions and annual fees and for medical device establishments. For fiscal year 2025, the small business user fee for a 510(k) premarket notification application is $9,280. If the FDA agrees that the device is substantially equivalent to a predicate device currently on the market, it will grant 510(k) clearance to commercially market the device. If the FDA determines that the device is “not substantially equivalent” to a previously cleared device, the device is automatically designated as a Class III device. The device sponsor must then fulfill more rigorous PMA requirements, or can request a risk-based classification determination for the device in accordance with the “de novo” process, which is a route to market for novel medical devices that are low to moderate risk and are not substantially equivalent to a predicate device.
After a device receives 510(k) marketing clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change or modification in its intended use, will require a new 510(k) clearance or, depending on the modification, PMA approval or de novo reclassification. The FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), a de novo request or a PMA in the first instance, but the FDA can review any such decision and disagree with a manufacturer’s determination. If the FDA disagrees with a manufacturer’s determination, the FDA can require the manufacturer to cease marketing and/or request the recall of the modified device until 510(k) marketing clearance or until PMA approval is obtained or a de novo request is granted. Also, in these circumstances, the manufacturer may be subject to significant regulatory fines or penalties.
Over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products. For example, in November 2018, the FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals included plans to potentially sunset certain older devices that were used as predicates under the 510(k) clearance pathway, and to potentially publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. In May 2019, the FDA solicited public feedback on these proposals. The FDA requested public feedback on whether it should consider certain actions that might require new authority, such as whether to sunset certain older devices that were used as predicates under the 510(k) clearance pathway. These proposals have not yet been finalized or adopted, and the FDA may work with Congress to implement such proposals through legislation.
More recently, in September 2019, the FDA finalized guidance describing an optional “safety and performance based” premarket review pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k) clearance pathway by showing that such device meets objective safety and performance criteria established by the FDA, thereby obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. The FDA intends to develop and maintain a list of device types appropriate for the “safety and performance based” pathway and will continue to develop product-specific guidance documents that identify the performance criteria for each such device type, as well as the testing methods recommended in the guidance documents, where feasible.
PMA Approval Pathway
41
If any of our products are classified as Class III, they will be subject to a PMA approval process. At this time, we believe, but cannot be certain, that our devices will be approved under Class II, thus avoiding the time consuming and expensive PMA approval pathway. Class III devices require PMA approval before they can be marketed, although some pre-amendment Class III devices for which the FDA has not yet required a PMA are cleared through the 510(k) process. The PMA process is more demanding than the 510(k) premarket notification process. In a PMA, the manufacturer must demonstrate that the device is safe and effective, and the PMA must be supported by extensive data, including data from preclinical studies and human clinical trials. The PMA must also contain a full description of the device and its components, a full description of the methods, facilities and controls used for manufacturing and proposed labeling. Following receipt of a PMA, the FDA determines whether the application is sufficiently complete to permit a substantive review. If the FDA accepts the application for review, it has 180 days under the FDCA to complete its review of a PMA, although in practice, the FDA’s review often takes significantly longer, and can take up to several years. An advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. The FDA may or may not accept the panel’s recommendation. In addition, the FDA will generally conduct a pre-approval inspection of the applicant or its third-party manufacturers’ or suppliers’ manufacturing facility or facilities to ensure compliance with the QSR.
The FDA will approve the new device for commercial distribution if it determines that the data and information in the PMA constitute valid scientific evidence and that there is reasonable assurance that the device is safe and effective for its intended use(s). The FDA may approve a PMA with post-approval conditions intended to ensure the safety and effectiveness of the device, including, among other things, restrictions on labeling, promotion, sale and distribution and collection of long-term follow-up data from patients in the clinical study that supported PMA approval or requirements to conduct additional clinical studies post-approval. The FDA may condition PMA approval on some form of post-market surveillance when deemed necessary to protect the public health or to provide additional safety and efficacy data for the device in a larger population or for a longer period of use. In such cases, the manufacturer might be required to follow certain patient groups for a number of years and to make periodic reports to the FDA on the clinical status of those patients. Failure to comply with the conditions of approval can result in material adverse enforcement action, including withdrawal of the approval.
Certain changes to an approved device, such as changes in manufacturing facilities, methods or quality control procedures, or changes in the design performance specifications, which affect the safety or effectiveness of the device, require submission of a PMA supplement. PMA supplements often require submission of the same type of information as a PMA, except that the supplement is limited to information needed to support any changes from the device covered by the original PMA and may not require as extensive clinical data or the convening of an advisory panel. Certain other changes to an approved device require the submission of a new PMA, such as when the design change causes a different intended use, mode of operation, and technical basis of operation, or when the design change is so significant that a new generation of the device will be developed, and the data that were submitted with the original PMA are not applicable for the change in demonstrating a reasonable assurance of safety and effectiveness. We do not currently expect any of our products to be marketed pursuant to a PMA.
Clinical Trials
Clinical trials are almost always required to support a PMA and are sometimes required to support a 510(k) submission. All clinical investigations of devices to determine safety and effectiveness must be conducted in accordance with the FDA’s investigational device exemption (“IDE”) regulations which govern investigational device labeling, prohibit promotion of the investigational device and specify an array of recordkeeping, reporting and monitoring responsibilities of study sponsors and study investigators. If the device presents a “significant risk” to human health, as defined by the FDA, the FDA requires the device sponsor to submit an IDE application to the FDA, which must become effective prior to commencing human clinical trials. If the device under evaluation does not present a significant risk to human health, then the device sponsor is not required to submit an IDE application to the FDA before initiating human clinical trials, but must still comply with abbreviated IDE requirements when conducting such trials. A significant risk device is one that presents a potential for serious risk to the health, safety or welfare of a patient and either is implanted, used in supporting or sustaining human life, substantially important in diagnosing, curing, mitigating or treating disease or otherwise preventing impairment of human health, or otherwise presents a potential for serious risk to a subject. An IDE application must be supported by appropriate data, such as animal and laboratory test results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The IDE will automatically become effective 30 days after receipt by the FDA unless the FDA notifies the Company that the investigation may not begin. If the FDA determines that there are deficiencies or other concerns with an IDE for which it requires modification, the FDA may permit a clinical trial to proceed under a conditional approval.
42
Regardless of the degree of risk presented by the medical device, clinical studies must be approved by, and conducted under the oversight of, an Institutional Review Board (“IRB”) for each clinical site. The IRB is responsible for the initial and continuing review of the IDE, and may pose additional requirements for the conduct of the study. If an IDE application is approved by the FDA and one or more IRBs, human clinical trials may begin at a specific number of investigational sites with a specific number of patients, as approved by the FDA. If the device presents a non-significant risk to the patient, a sponsor may begin the clinical trial after obtaining approval for the trial by one or more IRBs without separate approval from the FDA, but must still follow abbreviated IDE requirements, such as monitoring the investigation, ensuring that the investigators obtain informed consent and labeling and record-keeping requirements. Acceptance of an IDE application for review does not guarantee that the FDA will allow the IDE to become effective and, if it does become effective, the FDA may or may not determine that the data derived from the trials support the safety and effectiveness of the device or warrant the continuation of clinical trials. An IDE supplement must be submitted to, and approved by, the FDA before a sponsor or investigator may make a change to the investigational plan that may affect its scientific soundness, study plan or the rights, safety or welfare of human subjects.
During a study, the sponsor is required to comply with the applicable FDA requirements, including, for example, trial monitoring, selecting clinical investigators and providing them with the investigational plan, ensuring IRB review, adverse event reporting, record keeping and prohibitions on the promotion of investigational devices or on making safety or effectiveness claims for them. The clinical investigators in the clinical study are also subject to FDA regulations and must obtain patient informed consent, rigorously follow the investigational plan and study protocol, control the disposition of the investigational device and comply with all reporting and recordkeeping requirements. Additionally, after a trial begins, we, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the risks to study subjects outweigh the anticipated benefits.
Post-Market Regulation
After a device is cleared or approved for marketing, numerous and pervasive regulatory requirements continue to apply. These include:
•establishment registration and device listing with the FDA;
•QSR requirements, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the design and manufacturing process;
•labeling regulations and FDA prohibitions against the promotion of investigational products, or the promotion of “off-label” uses of cleared or approved products;
•requirements related to promotional activities;
•clearance or approval of product modifications to 510(k)-cleared devices that could significantly affect safety or effectiveness or that would constitute a major change in intended use of one of our cleared devices, or approval of certain modifications to PMA-approved devices;
•medical device reporting regulations, which require that a manufacturer report to the FDA if a device it markets may have caused or contributed to a death or serious injury, or has malfunctioned and the device or a similar device that it markets would be likely to cause or contribute to a death or serious injury, if the malfunction were to recur;
•correction, removal and recall reporting regulations, which require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the FDCA that may present a risk to health;
•the FDA’s recall authority, whereby the agency can order device manufacturers to recall from the market a product that is in violation of governing laws and regulations; and post-market surveillance activities and regulations, which apply when deemed by the FDA to be necessary to protect the public health or to provide additional safety and effectiveness data for the device.
Our manufacturing processes are required to comply with the applicable portions of the QSR, which cover the methods and the facilities and controls for the design, manufacture, testing, production, processes, controls, quality assurance, labeling, packaging, distribution, installation and servicing of finished devices intended for human use. The QSR also requires, among other things, maintenance of a device master file, device history file and complaint files. As a
43
manufacturer, we will be subject to periodic scheduled or unscheduled inspections by the FDA. Our failure to maintain compliance with the QSR requirements could result in the shut-down of, or restrictions on, our manufacturing operations and the recall or seizure of our products, which would have a material adverse effect on our business. The discovery of previously unknown problems with any of our products, including unanticipated adverse events or adverse events of increasing severity or frequency, whether resulting from the use of the device within the scope of its clearance or off-label by a physician in the practice of medicine, could result in restrictions on the device, including the removal of the product from the market or voluntary or mandatory device recalls.
The FDA has broad regulatory compliance and enforcement powers. If the FDA determines that we failed to comply with applicable regulatory requirements, it can take a variety of compliance or enforcement actions, which may result in any of the following sanctions:
•warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties;
•recalls, withdrawals or administrative detention or seizure of our products;
•operating restrictions or partial suspension or total shutdown of production;
•withdrawing 510(k) clearances or PMA approvals that have already been granted;
•refusal to grant export approvals for our products; or
•criminal prosecution.
Healthcare Regulatory Laws
Within the U.S., our products and our customers will be subject to extensive regulation by a wide range of federal and state agencies that govern business practices in the medical device industry. These laws include federal and state anti-kickback, fraud and abuse, false claims, transparency and anti-corruption statutes and regulations. Internationally, other governments also impose regulations in connection with their healthcare reimbursement programs and the delivery of healthcare items and services.
U.S. federal healthcare fraud and abuse laws will generally apply to our activities, among other reasons because we expect that our products will be covered under federal healthcare programs such as Medicare and Medicaid. The U.S. federal healthcare program Anti-Kickback Statute (the “Anti‑Kickback Statute”) is particularly relevant because of its broad applicability. Specifically, the Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for, or to induce, either the referral of an individual, or the furnishing, arranging for or recommending a good or service for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. Almost any financial interaction with a healthcare provider, patient or customer will implicate the Anti-Kickback Statute. Statutory exceptions and regulatory safe harbors protect certain interactions if specific requirements are met. However, only those interactions that represent fair market value exchanges generally are protected by a safe harbor or exception. The government can exercise enforcement discretion in taking action against unprotected activities. Further, a person or entity does not need to have actual knowledge of the Anti-Kickback Statute or specific intent to violate it in order to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act or federal civil money penalties statute. Penalties for Anti-Kickback Statute violations may include both criminal penalties such as imprisonment and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. Exclusion would mean that diagnostic tests using our products would no longer be eligible for reimbursement under federal healthcare programs.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of patients for healthcare items or services reimbursed by any payor, not only federal healthcare programs. Insurance companies may also bring a private cause of action for treble damages against a manufacturer for a pattern of causing false claims to be filed under the federal Racketeer Influenced and Corrupt Organizations Act.
Another development affecting the healthcare industry is the increased use of the federal Civil False Claims Act and, in particular, actions brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has
44
submitted a false claim to the federal government, and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted false claim laws analogous to the Civil False Claims Act, although many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program.
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), among other things, created two new federal crimes: healthcare fraud and false statements relating to healthcare matters. The HIPAA healthcare fraud statute prohibits, among other things, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private payors. A violation of this statute is a felony and may result in fines, imprisonment and/or exclusion from government-sponsored programs. The HIPAA false statements statute prohibits, among other things, knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation in connection with the delivery of or payment for healthcare benefits, items or services. A violation of this statute is a felony and may result in fines and/or imprisonment. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statutes or specific intent to violate them in order to have committed a violation.
Laws and regulations have also been enacted by the federal government and various states to regulate the sales and marketing practices of medical device and pharmaceutical manufacturers. The laws and regulations generally limit financial interactions between manufacturers and healthcare providers, require pharmaceutical and medical device companies to comply with voluntary compliance standards issued by industry associations and the relevant compliance guidance promulgated by the U.S. federal government and/or require disclosure to the government and/or public of financial interactions (so-called “sunshine laws”). Many of these laws and regulations contain ambiguous requirements or require administrative guidance for implementation. Manufacturers must adopt reasonable interpretations of requirements if there is ambiguity and those interpretations could be challenged. Given the lack of clarity in laws and their implementation, our activities could be subject to the penalty provisions of the pertinent federal and state laws and regulations.
Coverage and Reimbursement
Over the past few years, the growth rate of advanced imaging volumes has slowed in part due to additional patient-related cost-sharing programs and an increasing trend of third-party payors intensifying their utilization management efforts, for example, through benefit managers who require prior authorizations to control the growth rate of imaging services generally. We expect that these trends will continue.
By way of example, in the U.S., the Protecting Access to Medicare Act of 2014 required CMS, in conjunction with medical specialty societies, to adopt appropriate use criteria (“AUC”) for certain advanced diagnostic imaging services, including MRI, CT, nuclear medicine (including PET). Beginning in 2020, payment is made to the furnishing professional for an applicable advanced diagnostic imaging service only if the claim indicates that the ordering professional consulted a qualified clinical decision support mechanism, as identified by the Department of Health and Human Services (“HHS”), as to whether the ordered service adheres to the applicable AUC. Applicable settings include physician offices, hospital outpatient departments, including emergency departments, ambulatory surgical centers and independent diagnostic testing facilities. Advanced imaging services ordered by certain physicians identified as having outlier-ordering partners will be subject to prior authorization for applicable imaging services provided to Medicare beneficiaries. The outlier methodology used by CMS will be subject to future notice and comment rulemaking before the prior authorization component is implemented. We cannot predict the full impact of this project.
Third-party payors may impose limits on coverage or reimbursement for diagnostic imaging services, including denying reimbursement for tests that do not follow recommended diagnostic procedures or can only be billed using an unlisted or miscellaneous code. To the extent our customers will depend on third-party payors, unfavorable coding, coverage and reimbursement policies may constrict the profit margins of our provider customers, which may force us to lower our fees to attract and retain customers. If we are required to request new billing codes that more precisely identify and describe our imaging services, coverage is limited or reimbursement rates are inadequate, a healthcare provider might find it financially unattractive to own diagnostic imaging systems. It is possible that third-party payor coding, coverage and reimbursement policies will affect the need or prices for our products in the future, which could significantly affect our financial performance and our ability to conduct our business.
Healthcare Reform
In the U.S. and certain foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system. In March 2010, the Patient Protection and Affordable Care Act,
45
and the Health Care and Education Reconciliation Act of 2010 (collectively, the “ACA”) was signed into law and substantially changed the way healthcare is financed by both governmental and private insurers in the United States. The ACA contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement adjustments and fraud and abuse changes. Additionally, the ACA imposed, among other things, a new federal excise tax on the sale of certain medical devices, which, through a series of legislative amendments, was suspended, effective January 1, 2016, and subsequently repealed altogether on December 20, 2019, provided incentives to programs that increase the federal government’s comparative effectiveness research and implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models. Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future.
Since its enactment, there have been judicial and Congressional challenges to certain aspects of the ACA, and we expect there will be additional challenges and amendments to the ACA in the future. By way of example, in 2017, Congress enacted the Tax Cuts and Jobs Act (the “TCJA”), which eliminated the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate.” On December 14, 2018, a Texas U.S. District Court Judge ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the TCJA, the remaining provisions of the ACA are invalid as well. On December 18, 2019, the U.S. Court of Appeals for the 5th Circuit ruled that the individual mandate was unconstitutional and remanded the case back to the District Court to determine whether the remaining provisions of the ACA are invalid as well. However, the decision of the U.S. Court of Appeals for the 5th Circuit was appealed to the U.S. Supreme Court. On June 17, 2021, the U.S. Supreme Court held that the states that initially commenced the challenge to the ACA didn’t have standing to challenge the law, effectively ending this challenge. But it remains possible that future challenges to the ACA may be brought, and it is unclear how any future decisions and other efforts to repeal and replace the ACA will impact the ACA.
Other legislative changes have been proposed and adopted in the United States since the ACA was enacted, including aggregate reductions of Medicare payments to providers of 2% per fiscal year and reduced payments to several types of Medicare providers. We believe the overall escalating cost of medical products and services being paid for by the government and private health insurance has led to, and will continue to lead to, increased pressures on the healthcare and medical device industry to reduce the costs of products and services.
Data Privacy and Security
Medical device companies may be subject to U.S. federal and state and foreign health information privacy, security and data breach notification laws, which may govern the collection, use, disclosure and protection of health-related and other personal information. In the U.S., HIPAA imposes privacy, security and breach reporting obligations with respect to individually identifiable health information upon “covered entities” (health plans, health care clearinghouses and certain health care providers), and their respective business associates, individuals or entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HIPAA and its respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. HIPAA mandates the reporting of certain breaches of health information to HHS, affected individuals and if the breach is large enough, the media. Entities that are found to be in violation of HIPAA as the result of a breach of unsecured protected health information (“PHI”), a complaint about privacy practices or an audit by HHS, may be subject to significant civil, criminal and administrative fines and penalties and/or additional reporting and oversight obligations if required to enter into a resolution agreement and corrective action plan with HHS to settle allegations of HIPAA non-compliance. The Health Information Technology and Clinical Health Act also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
Even when HIPAA does not apply, according to the Federal Trade Commission (the “FTC”), failing to take appropriate steps to keep consumers’ personal information secure constitutes unfair acts or practices in or affecting commerce in violation of Section 5(a) of the Federal Trade Commission Act, 15 U.S.C § 45(a). The FTC expects a company’s data security measures to be reasonable and appropriate in light of the sensitivity and volume of consumer information it holds, the size and complexity of its business, and the cost of available tools to improve security and reduce vulnerabilities. Individually identifiable health information is considered sensitive data that merits stronger safeguards. The
46
FTC’s guidance for appropriately securing consumers’ personal information is similar to what is required by the HIPAA Security Rule.
In addition, certain state and non-U.S. laws, such as the European Union’s General Data Protection Regulation (2016/679) (“GDPR”), govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Further, “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity, are also subject to certain HIPAA privacy and security standards. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties and private litigation. For example, California recently enacted legislation, the California Consumer Privacy Act (“CCPA”), which went into effect January 1, 2020. The CCPA, among other things, creates new data privacy obligations for covered companies and provides new privacy rights to California residents, including the right to opt out of certain disclosures of their information. The CCPA also creates a private right of action with statutory damages for certain data breaches, thereby potentially increasing risks associated with a data breach. Although the law includes limited exceptions, including for PHI maintained by a covered entity or business associate, it may regulate or impact our expected processing of personal information depending on the context. In Europe, the GDPR went into effect in May 2018 and introduces strict requirements for processing the personal data of European Union data subjects. Companies that must comply with the GDPR face increased compliance obligations and risk, including more robust regulatory enforcement of data protection requirements and potential fines for noncompliance of up to €20 million or 4% of the annual global revenues of the noncompliant company, whichever is greater. The State of Israel has also implemented data protection laws and regulations, including the Israeli Protection of Privacy Law of 1981.
Foreign Regulation
As we plan to market and deploy the QT Breast Scanner and products under development broadly across the globe, we will be subject to regulations applicable to medical and radiation-emitting devices in the jurisdictions in which we operate, which regulations vary among countries. While some countries’ regulations may not impose barriers to marketing and selling our products or only require certain notification, others may require that we obtain the clearance, registration or approval of a specified regulatory body. The process for obtaining such clearance, registration or approvals may involve additional testing and time. Furthermore, complying with foreign regulatory requirements can be expensive and time-consuming, and we will need to seek for regulatory clearances or approvals in each country in which we plan to market our products. In addition, depending on the country, if we modify our products, we may need to apply for additional regulatory clearances or approvals before we are permitted to sell the modified product. Also, for maintaining our authorizations in a particular country, we will need to continue meeting quality and safety standards required in such country. The Company may seek additional regulatory approvals outside of the U.S. but as of the date of this registration statement/prospectus, we do not have sufficient information to determine when, if ever, the Company will receive regulatory approval from any other jurisdictions.
Finally, while regulatory clearance or approval by the FDA does not ensure registration, clearance or approval by regulatory authorities in other countries, registration or regulatory clearance or approval in one country, or denial thereof, may have effects on the regulatory process in others.
Intellectual Property, Patents & Trademarks
QT Imaging has multiple U.S. and European patents and 5 registered U.S. trademarks. QT Imaging does not disclose its proprietary reconstruction algorithm technology. The details regarding this intellectual property is shown below.
The table below shows our utility patents and utility patent applications:
| JURISDICTION | NUMBER | TITLE | DATE FILED | DATE GRANTED | EXPIRATION DATE | OWNER | ||||||||||||||||||||||||||||||||
US | US8827 908B2 | APPARATUS FOR ULTRASOUND IMAGING | 6/30/2011 | 9/9/2014 | 6/29/2032 | QT Imaging, Inc. and Esaote SpA | ||||||||||||||||||||||||||||||||
US | US9392 994B2 | APPARATUS AND METHOD FOR ULTRASOUND IMAGING WITH CONTRAST AGENTS | 4/5/2011 | 7/19/2016 | 11/19/2034 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
US | US7771 360B2 | BREAST SCANNING SYSTEM | 4/8/2004 | 8/10/2010 | 6/10/2029 | QT Ultrasound LLC | ||||||||||||||||||||||||||||||||
47
| JURISDICTION | NUMBER | TITLE | DATE FILED | DATE GRANTED | EXPIRATION DATE | OWNER | ||||||||||||||||||||||||||||||||
US | US8366 617B2 | BREAST SCANNING SYSTEM | 5/14/2008 | 2/5/2013 | 11/18/2031 | QT Ultrasound LLC, CVUS Clinical Trials LLC | ||||||||||||||||||||||||||||||||
US | US7699 783B2 | METHOD FOR IMAGING AND TREATING A BREAST | 6/15/2005 | 4/20/2010 | 1/23/2027 | QT Ultrasound LLC | ||||||||||||||||||||||||||||||||
EP DE FR GB | EP1765 176B1 | METHOD OF IMAGING AND APPARATUS FOR IMAGING AND TREATING A BREAST | 6/16/2005 | 12/19/2012 | 6/16/2025 | Biotex Pharma Investments LLC | ||||||||||||||||||||||||||||||||
EP DE FR GB ES IT NL | EP2148 612B1 | BREAST SCANNING SYSTEM | 5/14/2008 | 1/6/2021 | 5/14/2028 | QT Ultrasound LLC | ||||||||||||||||||||||||||||||||
EP DE FR GB | EP1610 687B1 | BREAST SCANNING SYSTEM | 4/9/2004 | 1/23/2019 | 4/9/2024 | QT Ultrasound LLC | ||||||||||||||||||||||||||||||||
US | US1076 5402B2 | AUTOMATIC LATERALITY IDENTIFICATION FOR ULTRASOUND TOMOGRAPHY SYSTEMS | 11/23/2016 | 9/8/2020 | 12/1/2038 | QT Ultrasound LLC | ||||||||||||||||||||||||||||||||
US | US8246 543B2 | IMAGING METHOD UTILIZING ATTENUATION AND SPEED PARAMETERS IN INVERSE SCATTERING TECHNIQUES | 5/14/2008 | 8/21/2012 | 3/8/2031 | QT Ultrasound LLC, CVUS Clinical Trials LLC | ||||||||||||||||||||||||||||||||
EP | EP3843 627A4 | APPLICATION OF MACHINE LEARNING TO ITERATIVE AND MULTIMODALITY IMAGE RECONSTRUCTION | 8/30/2019 | PENDING | QT Imaging, Inc. | |||||||||||||||||||||||||||||||||
US | US1117 0544B2 | APPLICATION OF MACHINE LEARNING TO ITERATIVE AND MULTIMODALITY IMAGE RECONSTRUCTION | 8/30/2019 | 11/9/2021 | 8/30/2039 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
US | US1043 3818B2 | COLOR CODING AN IMAGE FOR IDENTIFYING ANATOMY USING QUANTITATIVE TRANSMISSION ULTRASOUND TOMOGRAPHY | 12/8/2017 | 10/8/2019 | 6/12/2038 | QT Ultrasound LLC | ||||||||||||||||||||||||||||||||
US | 18/888,547 | MEDICAL IMAGING TECHNIQUES INCLUDING ADAPTIVE RECONSTRUCTION | 9/18/2024 | PENDING | QT Imaging, Inc. | |||||||||||||||||||||||||||||||||
US | 18/955,499 | EVALUATION OF TOPOLOGICAL COMPLEXITY AND GENERATION OF QUANTITATIVE MARKERS IN MEDICAL IMAGES | 11/21/2024 | PENDING | QT Imaging, Inc. | |||||||||||||||||||||||||||||||||
The table below shows our registered U.S. trademarks and trademark applications.
| TRADEMARKS | SERIAL NO | REGISTRATION | FILING DATE | Published for Opposition | Registration date | |||||||||||||||||||||||||||||||||
QT ULTRASOUND | 86295291 | 4729168 | 5/29/2014 | 10/21/2014 | 4/28/2015 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
QTVIEWER | 5586707 | 87067439 | 6/10/2016 | 5/16/2017 | 10/16/2018 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
QTSCAN | 87129339 | 5851942 | 8/5/2016 | 5/23/5017 | 9/3/2019 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
QTBREASTHEALTH | 88059928 | 5991966 | 7/31/2018 | 9/24/2019 | 2/18/2020 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
VOLOGRAPHY | 90329042 | 7183396 | 11/19/2020 | 6/8/2021 | 10/3/2023 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||
QT IMAGING | 98800489 | In the publication period | 10/14/2024 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||||
Breast Acoustic CT | 98414186 | In the publication period | 2/21/2024 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||||
Breast ACT | 98414157 | In the publication period | 2/21/2024 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||||
Breast Acoustic Computed Tomography | 98414202 | In the publication period | 2/21/2024 | QT Imaging, Inc. | ||||||||||||||||||||||||||||||||||
48
The Company is monitoring research laboratories, commercial companies or universities developing ultra-low frequency transmitted sound imaging using inverse scattering image reconstruction. Therefore, the Company believes that its patent and proprietary position is currently substantial and a very valuable asset.
Employees
As of December 31, 2024, QT Imaging had 21 employees. Of these, 20 are full-time employees, 16 work in research, development, manufacturing, regulatory and operations, and 5 work in general and administrative capacities. 12 employees are located in Novato, CA. None of QT Imaging’s employees are represented by a labor union or are subject to a collective bargaining agreement.
Corporate and Available Information
Our corporate headquarters are located in Novato, California pursuant to a lease agreement that will expire on May 31, 2027. We believe that these facilities will be adequate for our near-term needs. If required, we believe that suitable additional or alternative space would be available in the future on commercially reasonable terms.
Our corporate website address is www.qtimaging.com. Information contained on our website is not a part of or incorporated by reference into this annual report or any other document we file with the SEC, and the inclusion of our website address in this annual report is inactive textual reference only.
Item 1A: Risk Factors
An investment in our common stock involves a high degree of risk. In addition to the other information contained in this annual report, including the matters addressed under the heading “Cautionary Statement Regarding Forward-Looking Statements” and “Summary of Risk Factors,” together with all of the other information contained in this annual report, including our consolidated financial statements and the related notes thereto and the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and in our other public filings, in evaluating our business. The risk factors described below are not intended to be exhaustive and are not the only risks facing us. Additional risks not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, results of operations and cash flows in future periods or are not identified because they are generally common to businesses. The occurrence of one of more of the events or circumstances described in these risk factors, along or in combination with other events or circumstances, could harm our business, financial condition, results of operations, and growth prospects. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment. The following discussion should be read in conjunction with the respective consolidated financial statements of the Company, and the notes to the consolidated financial statements included therein.
Risks Related to Our Business, Financial Condition, and Need for Additional Capital
We are a development-stage company with limited operating history and significant losses since inception which may make it difficult to evaluate prospects for our future viability and predict our future performance. We may never be able to effectuate our business plan or achieve any meaningful revenue or reach profitability.
We have a limited operating history and only a preliminary and unproven business plan upon which investors may evaluate our prospects. We have not yet demonstrated the commercial viability at scale of our breast imaging technology platform. The QT Breast Scanner is deployed at facilities in the United States and abroad, but we have not demonstrated scale of deployment and manufacturing necessary to achieve commercial viability despite having clearance from the FDA for breast imaging with the QT Breast Scanner. Even if we are able to do so, we may not be able to manufacture the QT Breast Scanner device at the costs needed to support our business model. Even if we are able to commercialize some of our products or product candidates, there can be no assurance that we will generate significant revenues or ever achieve profitability. We expect to continue to incur significant sales and marketing, research and development, regulatory and other expenses as we expand our marketing efforts to increase adoption of our products, expand existing relationships, obtain regulatory approvals for our product candidates, conduct clinical studies on our existing and planned product candidates and develop new product candidates or add new features to our existing products. There is no assurance that our distribution partners will succeed in selling and servicing devices in sufficient volumes to help the company meet its business plan, revenue objectives or profitability.
Furthermore, even if our technology and product become commercially viable and deployed at scale, we may not generate sufficient revenue necessary to support our business. We may never successfully stimulate market interest in our QT Breast Scanner in the near-to-mid-term at any level or at all, which may cause our business to fail. The medical
49
imaging industry is also highly competitive, and our technology, products, services or business models may not achieve widespread market acceptance. If we are unable to address any issues mentioned above, or encounter other problems, expenses, difficulties, complications, and delays in connection with the starting and expansion of our business, our entire business may fail, in which case you may lose your entire investment.
We have a history of net losses and negative cash flow from operations since inception and we expect such losses and negative cash flows from operations to continue in the foreseeable future. As of December 31, 2024 and 2023, we had working capital deficit of $4.9 million and $2.5 million, respectively, and an accumulated deficit of approximately $31.9 million and $17.8 million, respectively. For the years ended December 31, 2024 and 2023, we incurred net losses attributable to the Company of approximately $9.0 million and $6.1 million, respectively. For the years ended December 31, 2024 and 2023, we used cash in operations of $10.0 million and $2.7 million, respectively. We anticipate our losses will continue to increase from current levels because we expect to incur additional costs related to developing our business, including research and development costs, manufacturing costs, employee-related costs, costs of complying with government regulations, intellectual property development and prosecution costs, marketing and promotion costs, capital expenditures, general and administrative expenses, and costs associated with operating as a public company.
Our ability to generate revenue from our operations and ultimately achieve profitability will depend on factors including but not limited to whether we can complete the development and commercialization of our QT Breast Scanner breast imaging technology and our future products, whether we can manufacture the QT Breast Scanner and future products on a commercial scale in such amounts and at such costs as we anticipate, and whether we can achieve market acceptance of our products, services and business models. The net losses that we incur may fluctuate significantly from period to period. As a result of these increased expenditures, we will need to generate significant additional revenue in order to offset our operating expenses and achieve and sustain profitability. Accordingly, we may not achieve or maintain profitability, and we may continue to incur significant losses in the future. Even if we achieve profitability, we cannot be sure that we will remain profitable for any substantial period of time. If we do not achieve or sustain profitability, it will be more difficult for us to finance our business and accomplish our strategic objectives, either of which would have a material adverse effect on our business, financial condition, results of operations and prospects and may cause the market price of the common stock to decline.
We may not be able to successfully execute our business model.
We are a company with limited operating history and we may not have the necessary resources, expertise and experience to successfully execute our business model on a global scale, such as obtaining the necessary approvals or clearances from the regulatory agencies of our target markets. Our ability to execute our model is dependent on a number of factors, including the ability of our senior management team to execute our model, our ability to incentivize, train and support international distribution partners in different geographic regions, our ability to begin or maintain our pace of product development, manufacturing and commercialization, our ability to meet the changing needs of the medical imaging market, and the ability of our employees to perform at a high-level. If we are unable to execute our model, or if our model does not drive the growth that we anticipate, or if our market opportunity is not as large as we have estimated, it could adversely affect our business and our prospects.
We have a limited operating history. If we successfully commercially launch the QT Breast Scanner, products under development that are cleared by the FDA and other regulatory agencies, and they do not achieve widespread market acceptance, we will not be able to generate the revenue necessary to support our business.
We have a limited operating history and have no history of successfully marketing our breast-imaging technology, the QT Breast Scanner or any other product using our 3D transmission ultrasound technology. We may fail to generate significant interest in the QT Breast Scanner, or other imaging products using our technology. These and other factors, including the following, may affect the rate and level of market acceptance:
•effectiveness of the sales and marketing efforts of us, and our distribution partners;
•perception by medical professionals and patients of the convenience, safety, efficiency and benefits of the QT Breast Scanner or products under development using our technology, compared to competing methods of medical imaging;
•opposition from certain industry leaders, which may limit our ability to promote the QT Breast Scanner or products under development that are cleared by the FDA and other regulatory agencies, and to penetrate into the medical imaging market in the U.S. or other geographical areas;
50
•the level of commitment and support that we receive from our partners, such as cloud storage providers, as well as medical professionals such as radiologists;
•coverage determinations and reimbursement levels of third-party payors;
•the changing and volatile U.S. and global economic environments, including as a result of the COVID-19 pandemic;
•timing of market introduction of competing products, and the sales and marketing initiatives of such products;
•press and blog coverage, social media coverage, and other publicity and public relations factors by others; and
•lack of financing or other resources to successfully develop and commercialize our technology and implement our business plan.
If cleared or approved for marketing by the FDA or other regulatory agencies, depending on the approved clinical indication, the QT Breast Scanner and products under development will be competing with existing and future imaging products and similar offerings. The technology underlying the QT Breast Scanner may be perceived as inferior or inaccurate and patients may be unwilling to undergo medical screening using the QT Breast Scanner or other products under development using our technology. Moreover, patients and medical professionals may be unwilling to depart from the current medical imaging technology. Medical professionals tend to be slow to change their medical diagnostic practices because of perceived liability risks arising from the use of new technology or products, and they may not recommend medical imaging using the QT Breast Scanner or other products under development using our technology until there is long-term clinical evidence to convince them to alter or modify their existing imaging methods. Our efforts to educate patients, radiologists and other members of the medical community on the benefits of our products require significant resources and may not be successful. Our efforts to educate the marketplace may require more resources than are required by conventional technologies marketed by our competitors. In the event that the QT Breast Scanner or other products under development using our technology are the subject of guidelines, clinical studies or scientific publications that are unfavorable or damaging, or otherwise call into question their benefits, we may have difficulty in convincing market participants to adopt our products. In addition, medical professionals, patients, providers of medical imaging services and third-party payors may not adopt or reimburse the use of the QT Breast Scanner or products under development in the near term or at all. Among other things, we need radiologists or other medical professionals to be trained to read the images that the QT Breast Scanner generates, and there are a limited number of such professionals currently trained and even fewer who are capable of providing such training. If we are unable to have a sufficient number of trained readers, then clinics will be less likely to purchase QT Breast Scanners as they will have difficulty using them to provide services to patients.
If we are unable to achieve or maintain an adequate level of market acceptance, we may not generate significant revenue or become profitable and our business, financial condition, results of operations and prospects would be significantly harmed.
The success of our business model is subject to numerous risks and uncertainties.
We expect sales to hospitals, academic medical centers, cancer centers, and imaging centers to be our primary customers. We may not be successful in achieving these goals for various reasons, including:
•the process of manufacturing and deploying the QT Breast Scanner and our products under development is a complex, multi-step process that depends on factors outside our control, and could cause us to expend significant time and resources prior to earning associated revenues;
•the manufacturing cost of the QT Breast Scanner and our products under development may be higher than we expect, may increase significantly, or may increase at a higher rate than anticipated;
•the manufacturing of the QT Breast Scanner and our future products may take longer than we expected, and we may have insufficient manufacturing capacity and experience delays in manufacturing and deployment, which would have a negative impact on the timing of our revenues;
•deployment and full utilization of the QT Breast Scanner may not be achieved if insurance and other reimbursements and patient co-pays are not sufficient to defray costs incurred in providing and interpreting scans by hospital imaging centers, cancer centers or other women’s health-care centers that purchase our devices and services, and we may not be able to sustain these relationships unless our devices can be profitable to these providers;
51
•a QT Breast Scanner device may perform fewer scans per day than our estimates due to a number of factors, including low market acceptance rate, technical failures and downtime, service disruptions, outages or other performance problems, which would have a negative impact on our revenues and our ability to recover costs; and
•our customers may not be able to find or retain a sufficient number of radiologists to review the images generated by the QT Breast Scanner device especially as we deploy additional systems and the volume of scans increases. There are currently a limited number of such radiologist professionals currently trained and even fewer who are capable of providing such training. If we are unable to have a sufficient number of trained readers, then clinics will be less likely to purchase QT Breast Scanners as they will have difficulty using them to provide services to patients.
Any of the above factors may negatively affect the successful commercialization and implementation of our business model, causing our business to fail.
The proceeds received in the Business Combination and since then, in the November 2024 private placement issuance of stock (the “Private Placement”) and the February 2025 note financing, will only fund operations for a limited time and we will need to obtain additional financing to continue operations and execute our business plans. If we are unable to obtain such financing, we may be unable to complete the development and commercialization of our technology and our products and services.
Our operations have consumed substantial amounts of cash since inception. Our net losses attributable to the Company were $8,984,880 and $6,098,951 for the years ended December 31, 2024 and 2023, respectively. In addition, significant resources were invested in the development of our QT Breast Scanner breast imaging technology prior to the June 2012 acquisition of the assets of TechniScan, a currently inactive medical device company based in Utah. Following the purchase of the TechniScan assets, QT Ultrasound completed the clinical trials needed to obtain FDA clearance. Approximately $39 million was invested in TechniScan (including $15.2 million in grants from the U.S. National Institutes of Health). Approximately $87 million has been invested in the Company and its predecessors since 2012 to fund asset acquisitions, product development, clinical trials, and FDA clearances. In November 2024, we raised $1 million in new cash proceeds in the Private Placement to fund continuing operations. On February 26, 2025, the Company borrowed $10.1 million from Lynrock Lake Master Fund LP (“Lynrock Lake”), of which $4.6 million was used to repay long-term debt and the remaining $5.5 million will be used for working capital needs.
We anticipate that our future cash requirements will continue to be significant and we will need to obtain additional financing beyond that provided by the Business Combination, the Private Placement and the loan from Lynrock Lake to implement our business plan as described in this annual report as these amounts will only fund operations for a limited time. Specifically, we may need to raise additional funds to complete the manufacture, shipping, installation and deployment of the QT Breast Scanner breast imaging product, as well as to support the continued research and development of this product and the development of other imaging products and product candidates for infant and orthopedic imaging applications, and to build contingencies for unforeseen events. Such financings could include equity financing, which may be dilutive to stockholders, or debt financing, which would likely restrict our ability to borrow from other sources. In addition, such securities may contain rights, preferences or privileges senior to those of the rights of the stockholders of the Company. Additional funds may not be available when we need them, on terms attractive to us, or at all.
If adequate funds are not available on a timely basis, we may be required to curtail the development of our technology, products or services, or materially delay, curtail, reduce or terminate our research and development and commercialization activities. We could be forced to sell or dispose of our rights or assets. Any inability to raise adequate funds on commercially reasonable terms could have a material adverse effect on our business, financial condition, results of operation and prospects, including the possibility that a lack of funds could cause our business to fail and liquidate with little or no return to investors.
We may need to raise additional capital, which may not be available on favorable terms, if at all, and which may cause dilution to stockholders, restrict our operations or adversely affect our ability to operate our business.
Our ability to raise additional capital may be significantly affected by general market conditions, the market price of our common stock, our financial condition, uncertainty about the future commercial success of our products, regulatory developments, the status and scope of our intellectual property, any ongoing arbitration or litigation, our compliance with applicable laws and regulations and other factors, many of which are outside our control. We cannot be certain that we will be able to obtain additional financing on favorable terms or at all. If we are unable to obtain needed financing on acceptable terms, or otherwise, we may not be able to implement our business plan, which could have a material adverse effect on our
52
business, financial condition and results of operations, including a decline in the trading price of our common stock. Any additional equity financings could result in additional dilution to our then existing stockholders. In addition, we may enter into additional financings that restrict our operations or adversely affect our ability to operate our business and, if we issue equity, debt or other securities to raise additional capital or restructure or refinance our existing indebtedness, the new equity, debt or other securities may have rights, preferences and privileges senior to those of our existing stockholders.
Our ability to generate the amount of cash needed to pay interest and principal on any indebtedness and our ability to refinance all or a portion of our indebtedness or obtain additional financing depends on many factors beyond our control.
Our ability to make scheduled payments on, or to refinance our obligations under, any indebtedness depends on our financial and operating performance and prevailing economic and competitive conditions. Certain of these financial and business factors, many of which may be beyond our control, are described above.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay capital expenditures, sell assets, raise additional equity capital, or restructure our debt. However, there is no assurance that such alternative measures may be successful or permitted under the agreements governing our indebtedness and, as a result, we may not be able to meet our scheduled debt service obligations. Even if successful, actions taken to improve short-term liquidity to meet our debt service and other obligations could harm our long-term business prospects, financial condition, and results of operations.
We cannot guarantee that we will be able to refinance our indebtedness or obtain additional financing on satisfactory terms or at all, including due to existing guarantees on our assets or our level of indebtedness and the debt incurrence restrictions imposed by the agreements governing our indebtedness. Further, the cost and availability of credit are subject to changes in the economic and business environment. If conditions in major credit markets deteriorate, our ability to refinance our indebtedness or obtain additional financing on satisfactory terms, or at all, may be negatively affected.
Our debt agreements contain restrictions that may limit our flexibility in operating our business.
The loan agreement with Lynrock Lake and related documents contain, and instruments governing any future indebtedness of ours would likely contain, a number of covenants that will impose significant operating and financial restrictions on us, including restrictions on our ability to, among other things:
•create liens on certain assets;
•incur additional debt;
•consolidate, merge, sell or otherwise dispose of all or substantially all of our assets; and
•sell certain assets.
Our loan agreement with Lynrock Lake also contains two financial covenants. These financial covenants are (i) a minimum qualified cash covenant of $500,000, and (ii) a minimum shipments and associated revenue and accounts receivable covenant for each quarter with amounts of at least 80% of the forecasted amount in the Company’s previously announced distribution agreement. The Company is also required within twelve (12) months of the entry into the Credit Agreement (unless such time period is extended by Lynrock Lake in its reasonable discretion) to create, or cause to be created, a holding company structure reasonably satisfactory to Lynrock Lake.
Any of these restrictions could limit our ability to plan for or react to market conditions and could otherwise restrict corporate activities. Any failure to comply with these covenants could result in a default under our secured credit facility or instruments governing any future indebtedness of ours. Additionally, our obligations to Lynrock Lake are secured by substantially all of our assets. Upon a default, unless waived, Lynrock Lake could elect to foreclose on our assets pledged to it to secure our obligations under our credit agreement and force us into bankruptcy or liquidation. In addition, a default under our secured loan could trigger a cross default under agreements governing any future indebtedness. Our results of operations may not be sufficient to service our indebtedness and to fund our other expenditures, and we may not be able to obtain financing to meet these requirements. If we experience a default under our loan agreement with Lynrock Lake or instruments governing our future indebtedness, our business, financial condition, and results of operations may be adversely impacted.
53
We are highly dependent on the successful development, marketing and sale of our breast imaging device and on other products and product candidates which are still in the development stage.
Our breast imaging technology is the basis of our business. The QT Breast Scanner is currently deployed in a limited number of cancer and other health centers, and is undergoing field testing and broad acceptance is uncertain. As a result, the success of our business plan is highly dependent on acceptance of our products, and on our ability to develop, manufacture and commercialize the technology and related products and services and our failure to do so could cause our business to fail. As part of our effort to build the sales and marketing capabilities of the Company, on May 31, 2023, QT Imaging entered into the NXC Sales Agent Agreement, pursuant to which QT Imaging appointed NXC as the non-exclusive agent for the sale of the QT Breast Scanner in non-exclusive territories: the U.S., U.S. territories, and U.S. Department of Defense installations. Subsequently, effective June 10, 2024, we replaced the NXC Sales Agent Agreement with the NXC Distribution Agreement, and since then, on December 11, 2024, we and NXC entered into the Amended Distribution Agreement, which we further amended on March 28, 2025. Under the terms of the Amended Distribution Agreement, NXC shall provide to QT Imaging a Forecast of the anticipated purchases of QT Breast Scanners during the subsequent twelve month period. The Amended Distribution Agreement further provides that the Forecast for 2025 and 2026 shall be no less than the MOQs set forth in an exhibit to the Amended Distribution Agreement, by quarter and by year. Furthermore, all purchase orders from NXC shall be for no less than the MOQs, which NXC must order on the quarterly and annual basis as set forth in an exhibit to the Amended Distribution Agreement.
Successful commercialization of medical imaging devices is a complex and uncertain process, dependent on the efforts of management, manufacturers, local operators, integrators, medical professionals, third-party payors, as well as general economic conditions, among other factors. Any factor that adversely impacts the development and commercialization of our imaging technology or related products and services, including the QT Breast Scanner, will have a negative impact on our business, financial condition, results of operations and prospects. Some potential factors that could adversely impact the development and commercialization of our imaging technology or related products and services include:
•our inability to achieve sufficient market acceptance by hospitals and clinics, providers of medical imaging services, medical professionals such as radiologists, third-party payors, and others in the medical community;
•our inability to compete with existing medical imaging technology companies with ultrasound, mammography and MRI systems, who have well entrenched market-share worldwide and significantly more resources than we do;
•our inability to hire, train and retain qualified sales and marketing personnel;
•our inability to establish, maintain and expand our sales, marketing and distribution networks;
•our inability to obtain and/or maintain necessary regulatory approvals; and
•our inability to effectively protect our intellectual property.
Our inability to successfully obtain additional clearances or approval from the FDA and other regulatory agencies worldwide, and commercialize the QT Breast Scanner and related products and services, and/or successfully develop, secure clearances and approvals, and commercialize additional products or any enhancements to the products which we may develop would have a material adverse effect on our business, financial condition, results of operations and prospects.
Products utilizing our technology may need to be approved or cleared by the FDA and similar regulatory agencies worldwide. We may not receive additional clearances and approvals from the FDA for the QT Breast Scanner, or may be delayed in receiving the necessary approval or clearance for our future products, which would adversely affect business, financial condition, results of operations and prospects.
On October 31, 2018, the FDA granted the Company’s Breakthrough Device designation request (Q181785) for the QT Breast Scanner. Unlike traditional breast imaging modalities, the QT Breast Scanner has no radiation, no injections, and no compression, potentially offering new opportunities for earlier and more frequent screening for young women at high risk for breast cancer who have no available FDA-cleared screening options. The Company has the following regulatory clearances:
•“The QT Breast Scanner is for use as an ultrasonic imaging system to provide reflection-mode and transmission-mode images of a patient’s breast. The QT Breast Scanner software also calculates the breast fibroglandular volume and total breast volume. The device is not intended to be used as a replacement for screening mammography”—FDA 510(k) K162372 and K220933
54
•“The QT Breast Scanner Model 2000A satisfies the requirements of the Certification Mark of the ECM [CE Mark Certification of the European Union]—No. 0P210730.QTUTQ02”
The Company has initiated engagement with the FDA to determine the most appropriate regulatory pathway for its premarket submission and to align on the clinical studies necessary to support such submission. The regulatory review process is inherently iterative, and the FDA may provide additional feedback requiring modifications or further evidence in response to the Company's initial submission. There can be no assurance that the Company will obtain the necessary regulatory clearances or approvals in a timely or cost-effective manner, or at all. The process of seeking FDA clearance or approval is resource-intensive, time-consuming, and subject to significant uncertainty. Delays, unexpected costs, or failure to obtain such regulatory authorizations could materially and adversely affect the Company's ability to commercialize its technology and generate revenue. Furthermore, even if regulatory clearance or approval is obtained, the Company’s products will remain subject to extensive post-market regulatory requirements, including compliance with quality system regulations, post-market surveillance, and reporting obligations. Any failure to comply with these requirements, or the identification of previously unknown issues related to the approved product, manufacturing processes, or manufacturers, could result in administrative or judicial enforcement actions by regulatory authorities, including but not limited to warning letters, fines, injunctions, product recalls, market withdrawal, or civil and criminal penalties. Even if the products containing our technology receive the required regulatory clearance or approval, such products will remain subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, or previously unknown problems with any approved commercial products, manufacturers or manufacturing processes are discovered, we could be subject to administrative or judicially imposed sanctions. Even if we obtain FDA approval of our product candidates, or new indications for our products, market acceptance of our products in the healthcare community, including physicians, patients and third-party payors will depend on many factors, including, without limitation: our ability to provide incremental clinical and economic data that shows the safety and clinical efficacy and cost-effectiveness of, and patient benefits from, our products and product candidates; whether our products and product candidates are included on insurance coverage plans; the willingness and ability of patients and the healthcare community to adopt new technologies; the pricing and reimbursement of our products relative to other products; and the marketing and distribution support for our products and product candidates.
In addition, the cost of compliance with new laws or regulations governing our technology or future products could adversely affect our business, financial condition, results of operations and prospects. New laws or regulations may impose restrictions or obligations on us that could force us to redesign our technology or other future products or services, and may impose restrictions that are not possible or practicable to comply with, which could cause our business to fail. See “—Risks Related to Healthcare Industry Shifts and Government Regulation.”
Our industry is highly competitive and is subject to technological change, which may result in new products or solutions that are superior to our technology or other future products we may bring to market from time to time.
The medical imaging industry is rapidly evolving and subject to intense and increasing competition. To compete successfully and to be able to establish and maintain a competitive position in current and future technologies, we will need to demonstrate the advantages of our technology over well-established alternative solutions, products and technologies, such as HHUS, ABUS, mammography and MRI, as well as newer methods of medical imaging and early detection. We believe that effectively engaging market interest for the QT Breast Scanner can be challenging. To achieve this, we will need to raise or develop financial resources, technical expertise, marketing, distribution or support capabilities and we may not be successful in doing so. Many of our current and potential competitors have substantially greater financial, manufacturing, marketing and technical resources than we do. Larger competitors may have substantially larger sales and marketing operations than we or our partners have or plan to have and may have greater name recognition. This may allow those competitors to spend more time with potential customers and to focus on a larger number of potential customers, which would give them a significant advantage over the sales and marketing team we would use and our distributors in making sales.
Larger competitors may also have broader product lines, which enable them to offer customers bundled purchase contracts and quantity discounts. These competitors may have more experience than we have in research and development, marketing, manufacturing, preclinical testing, conducting clinical studies, obtaining FDA and foreign regulatory approvals and marketing approved products. Our competitors may discover technologies and techniques, or enter into partnerships and collaborations, to develop competing products that are more effective or less costly than our products or the products we may develop. There can be no assurance that other companies will not succeed in developing or marketing devices and products that are more effective than our technology or products or that would render our technology or products obsolete or noncompetitive. Academic institutions, government agencies, and other public and private research organizations may seek patent protection regarding potentially competitive products or technologies and may establish exclusive collaborative
55
or licensing relationships with our competitors. Our competitors may be better equipped than we are to respond to competitive pressures. Competition will likely intensify. To our knowledge at the time of filing this annual report, we are not aware of any technologies approved for primary screening clearance by the FDA except for various types of technology related to X-ray mammography.
We expect to depend on third parties to manufacture the QT Breast Scanner and products under development and to supply certain component parts. Our reliance on third-party manufacturers and suppliers involves certain risks that may result in, among others, increased costs, quality or compliance issues, or failure to timely manufacture the QT Breast Scanner and products under development, any of which could materially harm our business.
We expect to rely on third-party suppliers for the commercial production of the QT Breast Scanner and products under development. Our current ability to successfully produce the QT Breast Scanner is limited and if our attempts at commercialization and deployment are successful, we will need the resources of well-established contract manufacturers to manufacture the QT Breast Scanner and products under development at scale and have contracted with CMSC as described in this annual report. In addition, we are dependent on a number of key suppliers for components and sub-assemblies to be able to successfully manufacture the QT Breast Scanner and products under development in limited quantities, and any disruption in the supply of these components and sub-assemblies will have a material impact on our business. Our dependence on such third-party manufacturers and suppliers involves a number of risks, including:
•insufficient capacity or delays in meeting our demand;
•inadequate manufacturing yields, inferior quality and excessive costs;
•inability to manufacture products that meet the agreed upon specifications;
•inability to obtain an adequate supply of materials;
•trade restrictions and changes in tariffs;
•inability to comply with the relevant regulatory requirements for the manufacturing process;
•limited warranties on products supplied to us;
•inability to comply with our contractual obligations;
•potential increases in prices; and
•increased exposure to potential misappropriation of our intellectual property.
If any of our manufacturers or suppliers breach their agreements, are unable to meet their contractual or quality requirements, or become unwilling to perform for any reason, we may be unable, or may be unable in a timely manner, to locate alternative acceptable manufacturers or suppliers and enter into favorable agreements with them.
In addition, if we are required to change the manufacturer of a critical component of our products, we will be required to verify that the new manufacturer maintains facilities, procedures and operations that comply with our quality and applicable regulatory requirements, which could further impede our ability to manufacture our products in a timely manner. Transitioning to a new supplier could be time-consuming and expensive, may result in interruptions in our operations and product delivery, could affect the performance specifications of our products or could require that we modify the design of those systems. If the change in manufacturer results in a significant change to any product, a new clearance or approval from the FDA or similar international regulatory authorization may be necessary before we implement the change, which could cause substantial delays. The occurrence of any of these events could harm our ability to meet the demand for our products in a timely or cost-effective manner. See “—Risks Related to Healthcare Industry Shifts and Government Regulation.”
We may experience development or manufacturing problems and higher costs, or delays that could limit our revenue, if any, or increase our losses.
Developing manufacturing procedures for new products requires developing specific production processes for those products. Developing such processes could be time consuming, and any unexpected difficulty in doing so could delay the successful commercialization and deployment of the QT Breast Scanner and products under development. Moreover, difficulties associated with adapting our technology and product design to the proprietary process technology and design rules of outside manufacturers can lead to reduced yields. Since low yields may result from either design or process
56
technology failures, yield problems may not be effectively determined or resolved until an actual product exists that can be analyzed and tested to identify process sensitivities relating to the design rules that are used. As a result, yield problems may not be identified until well into the production process, and resolution of yield problems may require cooperation between our manufacturers and us. This risk could be compounded by any offshore location of CMSC, increasing the effort and time required to identify, communicate and resolve manufacturing yield problems. Manufacturing defects that we do not discover during the manufacturing or testing process may lead to costly product recalls. These risks may lead to increased costs or delayed product delivery, which would harm our profitability and customer relationships. Furthermore, our, our manufacturers’ or our suppliers’ production processes and assembly methods may have to change to accommodate any significant, future expansion of our manufacturing capacity, which may increase the manufacturing costs, delay production of our products, reduce our product margin, require supplemental filings with the FDA or other regulatory authorities, any of which may adversely impact our business. If we are unable to keep up with demand for our products by successfully manufacturing and shipping our products in a timely manner, our revenue could be impaired, and market acceptance for our products could be adversely affected.
We plan to do business globally, including in certain countries where we might have limited resources and would be subject to additional regulatory burdens and other risks and uncertainties.
We expect to do business globally, including in North America and certain countries in Asia, Europe, Africa and South America. Commercialization of the QT Breast Scanner and products under development in foreign markets, either directly or through third parties, is subject to additional risks and uncertainties, including:
•reimbursement and insurance coverage;
•our inability to find agencies, dealers or distributors in specific countries or regions;
•our inability to directly control commercial activities of third parties;
•limited resources to be deployed to a specific jurisdiction;
•the burden of complying with complex and changing regulatory, tax, accounting and legal requirements;
•different medical imaging practice and customs in foreign countries affecting acceptance in the marketplace;
•import or export licensing and other requirements that could impair our ability to compete in international markets or subject our company to liability if we violate such laws and regulations;
•longer accounts receivable collection times;
•longer lead times for shipping;
•language barriers for technical training;
•reduced protection of intellectual property rights in some foreign countries;
•foreign currency exchange rate fluctuations; and
•interpretations of contractual provisions governed by foreign laws in the event of a contract dispute.
Sales of the QT Breast Scanner and products under development in foreign markets could also be adversely affected by the imposition of governmental controls, political and economic instability, trade restrictions and changes in tariffs, any of which may adversely affect our business, financial condition, results of operations and prospects.
If in the future we are approved for and are otherwise able to commercialize any of our products or services, but are unable to obtain adequate reimbursement or insurance coverage from third-party payors, we may not be able to generate significant revenue, in which case we may need to obtain additional financing.
Coding and coverage determinations as well as reimbursement levels and conditions are important to the commercial success of an imaging product or offering. The future availability of insurance coverage and reimbursement for newly approved medical devices is highly uncertain, and our future business will be greatly impacted by the level of reimbursement provided by third-party payors. In the United States, third-party payors decide which imaging products and services they will cover, how much they will pay and whether they will continue reimbursement. Third-party payors may not cover or provide adequate reimbursement for imaging services using the QT Breast Scanner and our products under development, assuming we are able to fully develop and obtain all regulatory approvals and clearances to market it in the
57
United States or other geographies. To date, we have not had any discussions with any third-party payors, including any regulatory agencies administering any government funded healthcare programs, regarding the coding, coverage or reimbursement for imaging services using the QT Breast Scanner or other products under development. Accordingly, unless government and other third-party payors provide coverage and reimbursement for the use of our products and services, patients may not use them, which would cause investors to lose their entire investment. A primary trend in the United States healthcare industry and elsewhere is cost containment. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular products and services. Reimbursement may not be available, or continue to be available, for imaging services using the QT Breast Scanner, our Medical Scan as a Service, other products under development or any other products we may develop in the future. Even if reimbursement is available, such reimbursement may not be adequate. We also will be subject to foreign reimbursement policies in the international markets we expect to enter. Decisions by health insurers or other third-party payors in these markets not to cover, or to discontinue reimbursing, our products could materially and adversely affect our business. If such decisions are made, they could also have a negative impact on our ability to generate revenues, in which case we may need to obtain additional financing.
Recent changes in the United States related to payment policies for imaging procedures could have a negative impact on the utilization of our imaging services.
In the United States, over the past several years, the Centers for Medicare & Medicaid Services (“CMS”), the federal agency responsible for administering the Medicare program, has implemented numerous changes to payment policies for imaging procedures in both the hospital setting and non-hospital settings, which include physician offices and freestanding imaging facilities. Some of these changes have had a negative impact on utilization of imaging services. Examples of these changes include:
•limiting payments for imaging services in physician offices and free-standing imaging facility settings based upon rates paid to hospital outpatient departments;
•reducing payments for certain imaging procedures when performed together with other imaging procedures in the same family of procedures on the same patient on the same day in the physician office and free-standing imaging facility setting;
•making significant revisions to the methodology for determining the practice expense component of the Medicare payment applicable to the physician office and free-standing imaging facility setting which results in a reduction in payment; and
•revising payment policies and reducing payment amounts for imaging procedures performed in the hospital outpatient setting.
We also expect increased regulation and oversight of advanced diagnostic testing. One provision in the Protecting Access to Medicare Act requires CMS to develop AUC that professionals must consult when ordering advanced diagnostic imaging services MRI, CT, nuclear medicine (including position emission tomography) and other advanced diagnostic imaging services that the Secretary of HHS may specify). Beginning in 2020, payment will be made to the furnishing professional for an applicable advanced diagnostic imaging service only if the claim indicates that the ordering professional consulted a qualified clinical decision support mechanism, as identified by HHS, as to whether the ordered service adheres to the applicable AUC. To the extent that these types of changes have the effect of reducing the aggregate number of diagnostic medical imaging procedures performed in the United States, our business, results of operations, financial condition and cash flows would be adversely affected.
Billing complexities associated with obtaining payment or reimbursement may negatively affect our revenue, cash flow and profitability.
Billing for imaging services is complex. Payment is provided by individual patients and from a variety of payors, such as commercial insurance carriers, managed care organizations and governmental programs. Each payor typically has different billing requirements, and the billing requirements of many payors have become increasingly stringent.
Among the factors complicating our customers’ ability to bill and receive reimbursement from third-party payors are:
•disputes among payors as to which party is responsible for payment;
•disparity in coverage among various payors;
58
•disparity in information and billing requirements among payors; and
•incorrect or missing billing information, which is required to be provided by the ordering physician.
In addition, we may be required to seek new billing codes for imaging services using the QT Breast Scanner, and regulatory authorities may not approve the creation of separate codes. Additionally, even if we are successful, these billing codes or the payment amounts associated with such codes may change in the future.
Any key supplier or distribution agreements that we have established or may establish in the future may not be successful or we may otherwise not realize the anticipated benefits from these agreements. We do not control third parties with whom we have or may have agreements, and we will rely on them to achieve results which may be significant to us. In addition, any current or future agreements may place the development and commercialization of our technology outside our control, may require us to relinquish important rights or may otherwise be on terms unfavorable to us.
We have entered into certain key distribution and manufacturing agreements, and expect to enter into additional, key supplier and distribution agreements with respect to the research, development, manufacture and commercialization of our technology with different relevant industry participants, including, among others, manufacturers of sub-assemblies and boards, cloud storage providers, distribution partners engaged in selling, marketing and servicing our products in their respective countries, and others as we develop our products including integrators, radiologists, cloud storage and third-party payors. See “Business-Sales and Marketing.” We refer to these third parties that we have agreements with or engage with for future potential agreements as collaborators. For a discussion of the Company’s Approved Supplier List and engagements with suppliers, see “Business-Manufacturing.” Any future potential relationships with collaborators may require us to rely on external consultants, advisors, and experts for assistance in several key functions, including research and development, manufacturing, regulatory and intellectual property. We cannot and will not control these third parties, but we may rely on them to achieve results, which may be significant to us. Relying upon these collaborative arrangements for our technology subjects us to a number of risks, including:
•we may not be able to control the amount and timing of resources that our collaborators may devote to our technology;
•should a collaborator fail to comply with applicable laws, rules or regulations when performing services for us, we could be held liable for such violations;
•our collaborators may have a shortage of qualified personnel, particularly radiologists who can review the medical images generated by the QT Breast Scanner and products and services under development, especially as we deploy additional devices and new products and the volume of scans increases;
•we may be required to relinquish important rights, such as marketing and distribution rights;
•business combinations or significant changes in a collaborator’s business strategy may adversely affect a collaborator’s willingness or ability to complete its obligations under any arrangement;
•under certain circumstances, a collaborator could move forward with a competing product developed either independently or in collaboration with others, including our competitors;
•our current or future collaborators may utilize our proprietary information in a way that could expose us to competitive harm;
•our collaborators could obtain ownership or other control over intellectual property that is material to our business; and
•collaborative arrangements are often terminated or allowed to expire, which could delay the ability to commercialize our technology.
In addition, if disputes arise between us and any of our collaborators, it could result in the delay or termination of the development, manufacturing or commercialization of products containing our technology, lead to protracted and costly legal proceedings, or cause collaborators to act in their own interest, which may not be in our interest. As a result, the collaborative arrangements that we may enter into, may not achieve their intended goals.
If any of these scenarios materialize, they could have a material adverse effect on our business, financial condition, results of operations and prospects.
59
We also may have other future products where it is desirable or essential to enter into agreements with a collaborator who has greater financial resources or different expertise than us, but for which we are unable to find an appropriate collaborator or are unable to do so on favorable terms. If we fail to enter into such collaborative agreements on favorable terms, it could materially delay or impair our ability to develop and commercialize, and increase the costs of development and commercialization of, our technology.
Even if we obtain all necessary FDA approvals, our products and product candidates may not achieve or maintain market acceptance.
Even if we obtain FDA approval of our products and product candidates, market acceptance of our products in the healthcare community, including physicians, patients and third-party payors, will depend on many factors, including:
•our ability to provide incremental clinical and economic data that shows the safety and clinical efficacy and cost-effectiveness of, and patient benefits from, our products;
•the availability of alternative products;
•whether our products or the use thereof are included on insurance company formularies or coverage plans;
•the willingness and ability of patients and the healthcare community to adopt our technologies;
•customer demand;
•liability risks generally associated with the use of new product candidates;
•the training required to use new product candidates;
•perceived inadequacy of evidence supporting clinical benefits or cost-effectiveness over existing alternatives;
•the convenience and ease of use of our products relative to other treatment methods;
•the pricing and reimbursement of our products relative to other treatment methods; and
•the marketing and distribution support for our products.
Even if we obtain all necessary FDA approvals, our products may fail to achieve market acceptance. If our products achieve market acceptance, they may not maintain that market acceptance over time if competing products or technologies are introduced that are received more favorably or are more cost-effective. Failure to achieve or maintain market acceptance would limit our ability to generate revenue and would have a material adverse effect on our business, financial condition, results of operations and prospects.
The outcome of any future claims and litigation could have a material adverse impact on our business, financial condition and results of operations.
We may, from time to time, be subject to claims and may become party to litigation in the normal course of business, including class action lawsuits. Such claims and litigation proceedings may be brought by third parties, including our customers, competitors, advisors, service providers, partners or collaborators, employees, and governmental or regulatory bodies. The final outcome of these claims and litigation, including any settlements, may be significant and may differ substantially from our expectations. We may not be able to determine the amount of any potential losses and other costs we may incur due to the inherent uncertainties of litigation and settlement negotiations. In the event we are required or decide to pay amounts in connection with any claims or lawsuits, such amounts could be significant and could have a material adverse impact on our liquidity, business, financial condition and results of operations.
We could become subject to product liability claims, product recalls, and warranty claims that could be expensive, divert management’s attention and harm our business reputation and financial results.
Our business exposes us to potential liability risks that are inherent in the marketing and sale of products used in patient care. We may be held liable if the QT Breast Scanner or our products and services under development causes injury or death or is found otherwise unsuitable during usage. The QT Breast Scanner and products and services currently under development incorporate sophisticated components and computer software. Complex software can contain errors, particularly when first introduced. In addition, new products or enhancements may contain undetected errors or performance problems that, despite testing, are discovered only after installation. Patients could allege or possibly prove defects of our products or other products that integrate our technology.
60
A product liability claim, regardless of its merit or eventual outcome, could result in significant legal defense costs and divert management’s attention. Regardless of merit or eventual outcome, liability claims may result in:
•injury to our reputation;
•costs of related litigation and substantial monetary awards to patients and others;
•decreased demand for our products and services;
•loss of revenue; and
•the inability to commercialize future products.
Any of these outcomes may have an adverse effect on our business, financial condition and results of operations, and may increase the volatility of our share price.
The coverage limits of our insurance policies we may choose to purchase to cover related risks may not be sufficient to cover future claims. If sales of the QT Breast Scanner and other products and services under development suffer future product liability claims, we may be unable to maintain product liability insurance at satisfactory rates or with adequate amounts or at all. A product liability claim, any product recalls or excessive warranty claims, whether arising from defects in design or manufacture or otherwise, could negatively affect our sales or require a change in the design or manufacturing process, any of which could harm our relationship with our customers and partners, and have a material adverse impact on our reputation and business, financial condition, results of operations and prospects.
In addition, if the QT Breast Scanner or other products integrating our technology are defective, we, our future customers or partners may be required to notify regulatory authorities and/or to recall the products and discontinue any services See “ —Risks Related to Healthcare Industry Shifts and Government Regulation—Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.” Any recall would divert management’s attention and financial resources and harm our reputation with customers, patients, medical professionals and third-party payors. A recall involving the QT Breast Scanner or our products under development, would be particularly harmful to our business. The adverse publicity resulting from any of these actions could adversely affect the perception of our customers or partners. These investigations or recalls, especially if accompanied by unfavorable publicity, could result in our incurring substantial costs, losing revenues and damaging our reputation, each of which would harm our business, financial condition, results of operations and prospects.
We do not expect to carry any business interruption insurance or any other insurance (except for director and officer, property and product liability insurance). As a result, we may incur uninsured losses, increasing the possibility that you would lose your entire investment in our company.
Our products and services are in the medical imaging field and so may be subject to claims. We are not immune from product liability or other product claim risks, and we may not be able to maintain insurance on acceptable terms against such risks or that such insurance will be sufficient to protect us against potential claims or that insurance will be available in the future in amounts sufficient to protect us. A product liability claim or other claim, as well as any claims for uninsured liabilities or in excess of insured liabilities, could have a material adverse effect on our business, financial condition, results of operations and prospects.
The mishandling or the perceived mishandling of sensitive information, or the occurrence of data security breaches, could harm our business.
We expect that the QT Breast Scanner and our products under development will enable us to accumulate a significant amount of highly sensitive and/or confidential information, including medical images and other medical information. These images could be received by our customers or collaborators, such as radiologists and other professionals at cancer screening and other healthcare facilities, to increase the probability of early disease detection. While employee contracts generally contain standard confidentiality provisions, our employees, customers or collaborators may not properly handle or process sensitive or confidential data. The improper handling of sensitive or confidential data, or even the perception of such mishandling (whether or not valid), or other security lapses by us, our customers or collaborators, could reduce demand for such products or otherwise expose us to financial or reputational harm or legal liability.
61
In addition, any security breach, including personal data breaches, or incident, including cybersecurity incidents, that we experience could result in unauthorized access to, misuse of, or unauthorized acquisition of the sensitive or confidential information and data (including medical information), the loss, corruption, or alteration of this data, interruptions in our operations, or damage to our systems. Any such incidents could expose us to claims, litigation, regulatory or other governmental investigations, administrative fines and potential liability. An increasing number of digital platforms have disclosed breaches of their security, some of which have involved sophisticated and highly targeted attacks on portions of their services. Because the techniques used to obtain unauthorized access, disable or degrade service, or sabotage systems change frequently and often are not foreseeable or recognized until launched against a target, we may be unable to anticipate these techniques or to implement adequate preventative measures. If an actual or perceived breach of our security occurs, public perception of the effectiveness of our security measures and brand could be harmed and our results of operations could be negatively affected. Data security breaches and other incidents may also result from non-technical means (e.g., actions by employees or contractors). Any compromise of our security could result in a violation of applicable security, privacy or data protection, consumer and other laws, regulatory or other governmental investigations, enforcement actions, and legal and financial exposure, including potential contractual liability. Any such compromise could also result in damage to our reputation and a loss of confidence in our security and privacy or data protection measures. Any of these effects could materially and adversely affect our business, financial condition and results of operations.
Our business and operations would suffer in the event of computer system failures, cyber-attacks or deficiencies in our cyber-security.
Our ability to execute our business strategy depends, in part, on the continued and uninterrupted performance of our IT systems, which support our operations. Despite the implementation of security measures, our internal computer systems, and those of third parties on which we rely, are vulnerable to damage from, among others, computer viruses, malware, natural disasters, terrorism, war, telecommunication and electrical failures, cyber-attacks or cyber-intrusions over the internet, attachments to emails, persons inside our organization, or persons with access to systems inside our organization or similar disruptive problems. The risk of a security breach or disruption, particularly through cyber-attacks or cyber intrusion, including by computer hackers, foreign governments, and cyber terrorists, has generally increased as the number, intensity and sophistication of attempted attacks and intrusions from around the world have increased. If such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our product development programs. Any such security breach may compromise information stored on our networks and may result in significant data losses or theft of personally identifiable information. A cybersecurity breach could also hurt our reputation by adversely affecting the patients’ perception of the security of their information. A number of proposed and enacted federal, state and international laws and regulations obligate companies to notify individuals of security breaches involving particular personally identifiable information, which could result from breaches experienced by us or by third parties, including collaborators, vendors, contractors or other organizations with which we expect to form strategic relationships. In addition, a cybersecurity attack could result in other negative consequences, including disruption of our internal operations, increased cyber security protection costs, lost revenue, regulatory actions or litigations.
Cyber-attacks and security vulnerabilities could lead to reduced revenue, increased costs, liability claims, or harm to our competitive position.
Increased sophistication and activities of perpetrators of cyber-attacks have resulted in an increase in information security risks in recent years. Hackers develop and deploy viruses, worms, and other malicious software programs that attack products and services and gain access to networks and data centers. In addition to extracting sensitive information, such attacks could include the deployment of harmful malware, ransomware, denial-of-service attacks, social engineering and other means to affect service reliability and threaten the confidentiality, integrity and availability of information. The prevalent use of mobile devices also increases the risk of data security incidents. If we experience difficulties maintaining existing systems or implementing new systems, we could incur significant losses due to disruptions in our operations.
Additionally, these systems contain valuable proprietary and confidential information and may contain personal data of our customers. While we believe we have taken reasonable steps to protect such data, techniques used to gain unauthorized access to data and systems, disable or degrade service, or sabotage systems, are constantly evolving, and we may be unable to anticipate such techniques or implement adequate preventative measures to avoid unauthorized access or other adverse impacts to such data or our systems. In addition, some of our third-party service providers and partners also collect and/or store our sensitive information and our customers’ data on our behalf, and these service providers and partners are subject to similar threats of cyber-attacks and other malicious internet-based activities, which could also expose us to risk of loss, litigation, and potential liability. A security breach could result in disruptions of our internal systems and business applications, harm to our competitive position from the compromise of confidential business information, or subject us to liability under laws that protect personal data. Additionally, actual, potential or anticipated attacks may cause us to incur
62
increasing costs, including costs to deploy additional personnel and protection technologies, train employees, and engage third-party experts and consultants. Specifically, as cyber threats continue to evolve, we may be required to expend additional resources to continue to enhance our information security measures and/or to investigate and remediate any information security vulnerabilities. Any of these consequences would adversely affect our revenue and margins. Additionally, even if we purchase cybersecurity insurance coverage, we cannot be certain that such coverage will be adequate for data security liabilities actually incurred, will cover any indemnification claims against us relating to any incident, will continue to be available to us on economically reasonable terms, or at all, or that any insurer will not deny coverage as to any future claim. The successful assertion of one or more large claims against us that exceed available insurance coverage, or the occurrence of changes in our insurance policies, including premium increases or the imposition of large deductible or co-insurance requirements, could have a material adverse effect on our reputation, business, prospects, results of operations and financial condition.
We are exposed to data and cybersecurity risks that could result in data breaches, service interruptions, ransomware and demands, harm to our reputation, protracted and costly litigation or significant liability.
In connection with the products and services that we provide, we collect, use, store, transmit and otherwise process certain confidential, proprietary and sensitive information, including personally identifiable information and PHI of customers, employees and others. We rely on the efficient, uninterrupted and secure operation of complex information technology systems and networks to operate our business and securely store, transmit and otherwise process such information. In the normal course of business, we also share information with our service providers and other third parties. A failure to safeguard the integrity, confidentiality, availability and authenticity of personal information, customer data and our proprietary data from cyber-attacks, unauthorized access, fraudulent activity (e.g., check “kiting” or fraud, wire fraud or other dishonest acts), data breaches, ransomware and other security incidents that we, our third-party service providers or our customers may experience may lead to modification, destruction, loss of availability or theft of critical and sensitive data pertaining to us, our customers or other third parties. While we have taken extensive precautions to protect such confidential, proprietary and sensitive information, including personal information, these risks were heightened due to our remote workforce due to the COVID-19 pandemic, and there can be no assurance that such actions will be sufficient to prevent cyber-attacks or security breaches or mitigate all potential risks to our systems, networks and data, particularly with the recent proliferation of ransomware attacks around the world. All such protective measures, as well as additional measures that may be required to comply with rapidly evolving data privacy and security standards and protocols imposed by law, regulation, industry standards or contractual obligations, have and will continue to cause us to incur substantial expenses. Failure to timely upgrade or maintain computer systems, software and networks as necessary could also make us or our third-party service providers susceptible to breaches and unauthorized access and misuse. We may be required to expend significant additional resources to modify, investigate or remediate vulnerabilities or other exposures arising from data and cybersecurity risks.
Improper access to our or our third-party service providers’ systems or databases could result in the theft, publication, deletion or modification of confidential, proprietary or sensitive information, including personal information. An actual or perceived breach of our security systems or those of our third-party service providers may require notification under applicable data privacy regulations or contractual obligations. The accidental or unauthorized access to or disclosure, loss, destruction, disablement, corruption or encryption of, use or misuse of or modification of our, our customers’ or other third parties’ confidential, proprietary or sensitive information, including personal information, by us or our third-party service providers could result in significant fines, penalties, orders, sanctions and proceedings or actions against us by governmental bodies and other regulatory authorities, customers or third parties, which could materially and adversely affect our business, financial condition and results of operations. Any such proceeding or action, and any related indemnification obligations, could damage our reputation, force us to incur significant expenses in defense of such proceeding or action, distract our management, increase our costs of doing business or result in the imposition of financial liability.
Despite our efforts to ensure the integrity, confidentiality, availability, and authenticity of our proprietary systems and information, it is possible that we may not be able to anticipate or to implement effective preventive measures against all cyber threats. No security solution, strategy, or measures can address all possible security threats or block all methods of penetrating a network or otherwise perpetrating a security incident. The risk of unauthorized circumvention of our security measures or those of our third-party providers, customers and partners has been heightened by advances in computer and software capabilities and the increasing sophistication of hackers, including those operating on behalf of nation-state actors, who employ complex techniques involving the theft or misuse of personal and financial information, counterfeiting, “phishing” or social engineering incidents, account takeover attacks, denial or degradation of service attacks, malware, fraudulent payment and identity theft. Because the techniques used by hackers change frequently and are increasingly complex and sophisticated, and new technologies may not be identified until they are launched against a target, we and our
63
third-party service providers may be unable to anticipate these techniques or detect an incident, assess its severity or impact, react or appropriately respond in a timely manner or implement adequate preventative measures. Our systems are also subject to compromise from internal threats, such as theft, misuse, unauthorized access or other improper actions by employees, service provides and other third parties with otherwise legitimate access to our systems or databases. The latency of a compromise is often measured in months, but could be years, and we may not be able to detect a compromise in a timely manner.
Due to applicable laws and regulations or contractual obligations, we may also be held responsible for any failure or cybersecurity breaches attributed to our third-party service providers as they relate to the information that we share with them. Although we generally have agreements relating to data privacy and security in place with our third-party service providers, they are limited in nature and we cannot guarantee that such agreements will prevent the accidental or unauthorized access to or disclosure, loss, destruction, disablement, corruption or encryption of, use or misuse of or modification of confidential, proprietary or sensitive information, including personal information, or enable us to obtain reimbursement from third-party service providers in the event we should suffer incidents resulting in accidental or unauthorized access to or disclosure, loss, destruction, disablement or encryption of, use or misuse of or modification of confidential, proprietary or sensitive information, including personal information. In addition, because we do not control our third-party service providers and our ability to monitor their data security is limited, we cannot ensure the security measures they take will be sufficient to protect confidential, proprietary or sensitive information (including personal information).
Regardless of whether a security incident or act of fraud involving our solutions is attributable to us or our third-party service providers, such an incident could, among other things, result in improper disclosure of information, harm our reputation and brand, reduce the demand for our products and services, lead to loss of customer business or confidence in the effectiveness of our security measures, disrupt normal business operations or result in our systems or products and services being unavailable. In addition, such incidents may require us to spend material resources to investigate or correct the incident and to prevent future security incidents, expose us to uninsured liability, increase our risk of regulatory scrutiny, expose us to protracted and costly litigation, trigger indemnity obligations, result in damages for contract breach, divert the attention of management from the operation of our business and otherwise cause us to incur significant costs or liabilities, any of which could affect our financial condition, results of operations and reputation. Moreover, there could be public announcements regarding any such incidents and any steps we take to respond to or remediate such incidents, and if securities analysts or investors perceive these announcements to be negative, it could, among other things, have a substantial adverse effect on the price of our common stock. In addition, our remediation efforts may not be successful. Further, any adverse findings in security audits or examinations could result in reputational damage to us, which could reduce the use and acceptance of our solutions, cause our customers to cease doing business with us or have a significant adverse impact on our revenue and future growth prospects. Furthermore, even if not directed at us specifically, attacks on other financial institutions could disrupt the overall functioning of the financial system or lead to additional regulation and oversight by federal and state agencies, which could impose new and costly compliance obligations.
If we fail to maintain properly the integrity or availability of our data or successfully consolidate, integrate, upgrade or expand our existing information systems, or if our technology products do not operate as intended, our business could be materially and adversely affected.
Our business depends on the integrity and timeliness of the data we use to serve our members, customers and health care professionals and to operate our business. If the data we rely upon to run our businesses is found to be inaccurate or unreliable or if we fail to maintain or protect our information systems and data integrity effectively, we could experience failures in our health, wellness and information technology products; lose existing customers; have difficulty attracting new customers; experience problems in determining medical cost estimates and establishing appropriate pricing; have difficulty preventing, detecting and controlling fraud; have disputes with customers, physicians and other health care professionals; become subject to regulatory sanctions, penalties, investigations or audits; incur increases in operating expenses; or suffer other adverse consequences. The volume of health care data generated, and the uses of data, including electronic health records, are rapidly expanding. Our ability to implement new and innovative services, automate and deploy new technologies to simplify administrative processes and clinical decision making, price our products and services adequately, provide effective service to our customers and consumers in an efficient and uninterrupted fashion, provide timely payments to care providers, and report accurately our results of operations depends on the integrity of the data in our information systems. In addition, connectivity among technologies is becoming increasingly important and recent trends toward greater consumer engagement in health care require new and enhanced technologies, including more sophisticated applications for mobile devices. Our information systems require an ongoing commitment of significant resources to maintain, protect and enhance existing systems and develop new systems to keep pace with continuing changes in information processing technology, evolving systems and regulatory standards and changing customer preferences. We
64
periodically consolidate, integrate, upgrade and expand our information systems’ capabilities as a result of technology initiatives, recently enacted regulations, changes in our system platforms and integration of new business acquisitions. Our process of consolidating the number of systems we operate, upgrading and expanding our information systems’ capabilities, enhancing our systems and developing new systems to keep pace with continuing changes in information processing technology may not be successful. Failure to protect, consolidate and integrate our systems successfully could result in higher than expected costs and diversion of management’s time and energy, which could materially and adversely affect our results of operations, financial position and cash flows. Certain of our businesses sell and install software products which may contain unexpected design defects or may encounter unexpected complications during installation or when used with other technologies utilized by the customer. A failure of our technology products to operate as intended and in a seamless fashion with other products could materially and adversely affect our results of operations, financial position and cash flows. Uncertain and rapidly evolving U.S. federal and state, non-U.S. and international laws and regulations related to health data and the health information technology market may alter the competitive landscape or present compliance challenges and could materially and adversely affect the configuration of our information systems and platforms, and our ability to compete in this market.
If significant tariffs or other restrictions related to “trade wars” are placed on U.S. made products or any related counter-measures are taken by any of the countries in which we operate or expect to operate, our revenue and results of operations may be materially harmed.
We have entered into the Canon Manufacturing Agreement with CMSC for the manufacture of the QT Breast Scanner in Japan, and may also have agreements we expect to enter into with manufacturers and/or suppliers in Asia for the volume production of components, sub-assemblies or the full assembly of the QT Breast Scanner and other products under development. If significant tariffs or other restrictions are placed by the United States government on imports or any related counter-measures are taken by the countries in which we have such manufacturing and outsourcing agreements, our business, financial condition and results of operations may be materially harmed. Alternatively, we may seek to shift production outside of the affected countries subject to tariffs or other restrictions, resulting in significant costs and disruption to our operations and business. Our business could also be impacted by retaliatory trade measures taken by other countries in response to existing or future tariffs, causing us to raise prices or make changes to our operations, any of which could materially harm our business, financial condition and results of operations.
Our business may be impacted by changes in general economic conditions.
Our business is subject to risks arising from changes in domestic and global economic conditions, including adverse economic conditions in markets in which we operate, which may harm our business. For example, the COVID-19 pandemic caused significant volatility and uncertainty in U.S. and international markets. Similar volatility and uncertainty could result from the imposition of tariffs and other trade restrictions intended to have an impact on global supply chains, which may harm our business. If our manufacturers and supplies cannot supply us with materials and product at the cost that we have planned upon, we could see increased expense in the production of the QT Breast Scanner. If our future customers significantly reduce spending in areas in which our technology and products are utilized, or prioritize other expenditures over our technology and products, our business, financial condition, results of operations and prospects would be materially adversely affected.
Disruption to the global economy could also result in a number of follow-on effects on our business, including a possible slow-down resulting from lower customer expenditures; inability of customers to pay for products, solutions or services on time, if at all; more restrictive export regulations which could limit our potential customer base; negative impact on our liquidity, financial condition and share price, which may impact our ability to raise capital in the market, obtain financing and secure other sources of funding in the future on terms favorable to us.
In addition, the occurrence of catastrophic events, such as hurricanes, storms, earthquakes, tsunamis, floods, medical epidemics and other catastrophes that adversely affect the business climate in any of our markets could have a material adverse effect on our business, financial condition and results of operations.
Technological change may adversely affect sales of our products and may cause our products to become obsolete.
The medical device market is characterized by extensive research and development, and rapid technological change. Technological progress or new developments in our industry could adversely affect clinical adoption of QT Breast Scanner and our other products under development, which could be rendered obsolete because of future innovations by our competitors with traditional methods like MRI, HHUS or mammography. We may be limited by resources, including qualified personnel, funds for capital investments, and other constraints from offering improvements to our products and services and our business, operating results and financial condition will suffer as a result.
65
Employee attrition may have an adverse impact on our business, results of operations or internal controls.
Our ability to attract, retain and develop qualified and experienced employees, including key executives and other talent, is critical for us to meet our business objectives. We compete with many other businesses to attract and retain employees. It is possible that we could experience loss of key personnel for a variety of causes. If we do not adequately plan for succession of key roles or if we are not successful in attracting or retaining new talent, our performance or internal control over financial reporting could be adversely impacted.
We plan to expand our operations and may not be able to manage our growth effectively, which could strain our resources and delay or derail implementation of our business objectives.
We will need to significantly expand our operations to implement our longer-term business plan and growth strategies, including building and expanding our internal organizational infrastructure to manage the regulatory approval process with the FDA for our product candidates. We will also be required to manage and form new relationships with various strategic partners, technology licensors, customers, manufacturers and suppliers, consultants and other third parties. This expansion and these new relationships will require us to significantly improve or replace our existing managerial, operational and financial systems, and procedures and controls; to improve the coordination between our various corporate functions; and to manage, train, motivate and maintain a growing employee base. The time and costs to effectuate these steps may place a significant strain on our management personnel, systems and resources, particularly if there are limited financial resources and skilled employees available at the time. We cannot assure that we will institute, in a timely manner or at all, the improvements to our managerial, operational and financial systems, procedures and controls necessary to support our anticipated increased levels of operations and to coordinate our various corporate functions, or that we will be able to properly manage, train, motivate and retain our anticipated increased employee base. If we cannot manage our growth initiatives, we will be unable to commercialize our products on a large-scale in a timely manner, if at all, and our business could fail.
If we do not manage our growth or control costs related to growth, our financial condition, results of operations and future growth prospects will suffer.
Our existing systems, facilities, procedures and personnel may not be adequate to support our future growth and operations. We intend to grow our business by expanding our customer base, sales force, and product offerings. Growth could place significant strain on our management, employees, operations, financial systems, and other resources. To accommodate significant growth, we could be required to open additional facilities, expand and improve or information systems and procedures, and hire, train, motivate and manage a growing workforce, all of which would increase our costs. Further, we may not succeed in our plans to accelerate or manage growth by expanding operations, personnel and other resources, or achieve results that are timely and profitable.
If we are unable to anticipate or keep pace with changes in the marketplace and the direction of technological innovation and customer demands, our technology may become less useful or obsolete and our operating results and financial condition will suffer.
Companies offering traditional medical imaging systems, such as General Electric, Siemens, Philips, Hologic, Varian, Fuji, Canon and Hitachi, are better established in the market than we are, have greater corporate, financial, operational, sales and marketing resources than we do, or have more experience in research and development than we have. Successful developments by these companies using 3D ultralow frequency transmitted sound imaging or other technologies by competitors resulting in new approaches for medical imaging, including technologies, products or services that are more effective or commercially attractive, could make our technology less useful or obsolete. We may also face opposition from certain industry leaders, who may have political influence and the ability to delay deployment of QT Breast Scanner and other products under development in certain geographical areas.
Our competitive position also depends on our ability to:
•generate widespread awareness, acceptance and adoption of our technology and future products or services;
•develop new or enhanced technologies or features that improve the convenience, efficiency, safety or perceived safety, and productivity of our technology and future products or services;
•properly identify customer needs and deliver new products or services or product enhancements to address those needs;
•limit the time required from prototype development to commercial production;
66
•limit the timing and cost of regulatory approvals;
•attract and retain qualified personnel and collaborators;
•protect our inventions with patents or otherwise develop proprietary products and processes; and
•secure sufficient capital resources to expand both our continued research and development, and sales and marketing efforts.
If our technology is not, or our future products or services are not, competitive based on these or other factors, our business would be harmed.
We may be unable to sustain revenue growth or profitability.
Our ability to increase revenue in future periods will depend on our ability to successfully penetrate our target markets and increase sales of our products which will, in turn, depend in part on our success in growing our customer base and obtaining reorders from those customers. New products and services will also need to be developed and approved or cleared by the FDA and foreign regulatory agencies. Our ability to become profitable and sustain profitability is highly dependent on our ability to sustain revenue growth and to successfully manage our costs. We are also subject to potential headwinds—adverse economic conditions in the markets we serve, political turmoil, pandemic and disease, acts of God, and other unforeseen factors beyond our control that may affect our ability to sustain revenue and profitability.
Our marketing efforts, including any social media marketing efforts that we may implement in the future, may expose our company to additional regulatory scrutiny, including from the FTC and other consumer protection agencies and regulators.
In addition to the laws and regulations enforced by the FDA, advertising for various services and for non-restricted medical devices is subject to federal truth-in-advertising laws enforced by the FTC, as well as comparable state consumer protection laws. The Company’s efforts to promote its prescription products via direct-to-consumer marketing and social media initiatives may subject us to additional scrutiny of its practices. For example, the FTC and other consumer protection agencies scrutinize all forms of advertising (whether in digital or traditional formats) for business services, consumer-directed products, and non-restricted medical devices to ensure that advertisers are not making false, misleading or unsubstantiated claims or failing to disclose material relationships between the advertiser and its products’ endorsers, among other potential issues. The FDA oversees the advertising and promotional labeling for restricted medical devices and ensures, among other things, that there is effective communication of, and a fair and balanced presentation of, the risks and benefits of such high-risk medical devices.
Under the Federal Trade Commission Act (“FTC Act”), the FTC is empowered, among other things, to (a) prevent unfair methods of competition and unfair or deceptive acts or practices in or affecting commerce; (b) seek monetary redress and other relief for conduct injurious to consumers; and (c) gather and compile information and conduct investigations relating to the organization, business, practices, and management of entities engaged in commerce. The FTC has very broad enforcement authority, and failure to abide by the substantive requirements of the FTC Act and other consumer protection laws can result in administrative or judicial penalties, including civil penalties, injunctions affecting the manner in which the Company would be able to market services or products in the future, or criminal prosecution. Any plans to increase our advertising activities that may be subject to these federal and state truth-in-advertising laws. Any actual or perceived non-compliance with those laws could lead to an investigation by the FTC or a comparable state agency, or could lead to allegations of misleading advertising by private plaintiffs. Any such action against us would disrupt the Company’s business operations, cause damage to our reputation, and result in material adverse effects on our business and financial performance.
The FDA requires that promotional labeling be truthful and not misleading, including with respect to any comparative claims made about competing products or technologies. In addition to FDA implications, the use of comparative claims also present risk of a lawsuit by the competitor under federal and state false advertising and unfair competition statutes (e.g., the Lanham Act) or unfair and deceptive trade practices law, and possibly also state libel law. Such a suit may seek injunctive relief against further advertising, a court order directing corrective advertising, and compensatory and punitive damages where permitted by law. Further, notwithstanding the ultimate outcome of any Lanham Act or similar complaint, the Company’s reputation and relationship with certain customers or distribution partners may be harmed as a result of the allegations related to its products or its business practices more generally.
67
Risks Related to Healthcare Industry Shifts and Government Regulation
We are subject to extensive government regulation, which could restrict the development, marketing, sale and distribution of our products and services, and could cause us to incur significant costs.
The Company’s ultrasound imaging products and associated services are subject to extensive pre-market and post-market regulation by the FDA and various other federal, state, local and foreign government authorities. Government regulation of medical devices is meant to assure their safety and effectiveness, and includes requirements for, among other things: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance and approval; conformity assessment procedures; record keeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to occur, could lead to death or serious injury; post-market approval studies; and product import and export.
The Company is also subject to numerous post-marketing regulatory requirements, which include quality system regulations related to the manufacture of the Company’s devices, labeling regulations and medical device reporting regulations. The last of these regulations requires us to report to the FDA if our devices cause or contribute to a death or serious injury, or malfunction in a way that would likely cause or contribute to a death or serious injury if the malfunction recurred. If we fail to comply with present or future regulatory requirements that are applicable, it may subject our company to enforcement action by the FDA, such as: warning or untitled letters; fines; injunctions; consent decrees; civil penalties; customer notifications; termination of distribution; recalls or seizures of products; administrative detention of medical devices believed to be adulterated or misbranded; delays in the introduction of products into the market; operating restrictions; total or partial suspension of production; refusal to grant future clearances or approvals for new products, new intended uses or modifications to our products; withdrawals or suspensions of current approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal prosecution or penalties. The occurrence of any of these events may have a material adverse effect on the Company’s business, financial condition and results of operations.
The laws and regulations to which the Company and its products are subject are complex and subject to periodic changes. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. See “Business-Government Regulation” for a more detailed description of laws and regulations that affect our business and operations.
Failure to comply with applicable regulation in the United States and in the countries where we will sell and distribute our products could harm our business.
QT Breast Scanner and other future products we develop are subject to extensive regulation in the U.S. and elsewhere, including by the FDA and its foreign counterparts, the U.S. Department of Justice (the “DOJ”) and the U.S. Health and Human Services-Office of the Inspector General (the “OIG”). The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance and approval; conformity assessment procedures; record keeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to occur, could lead to death or serious injury; post-market approval studies; and product import and export.
The regulations for products like QT Breast Scanner, products under development and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales for any approved product. Failure to comply with applicable regulations could jeopardize our ability to sell our future products, if cleared or approved, and result in enforcement actions such as: warning or untitled letters; fines; injunctions; consent decrees; civil penalties; customer notifications; termination of distribution; recalls or seizures of products; administrative detention of medical devices believed to be adulterated or misbranded; delays in the introduction of products into the market; operating restrictions; total or partial suspension of production; refusal to grant future clearances or approvals for new products, new intended uses or modifications to our products; withdrawals or suspensions of current approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal prosecution or penalties. The occurrence of any of these events would have a material adverse effect on our business, financial condition and results of operations and could result in stockholders losing their entire investment.
68
We may not receive, or may be delayed in receiving, the necessary clearances or approvals for our future products, and failure to timely obtain necessary clearances or approvals for our future products would adversely affect our ability to grow our business.
See “Business-Government Regulation” for a more detailed description of laws and regulations that affect our business and operations. In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the FDCA or approval of a PMA from the FDA, unless an exemption applies. Clinical data are sometimes required to support a pre-market approval application. In the process of obtaining PMA approval, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices. While we do not expect our products to be marketed under a PMA, should the FDA require we submit to a PMA approval process for any of our products, our business could suffer due to increased costs and timelines to receive such approvals.
If the FDA requires us to submit new 510(k) notifications or PMA applications for modifications to our previously cleared products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
The FDA can delay, limit or deny clearance or approval of a medical device for many reasons, including:
•our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory entity or notified body that our product candidates are safe or effective for their intended uses;
•the disagreement of the FDA or the applicable foreign regulatory body with the design or implementation of our clinical trials or the interpretation of data from pre-clinical studies or clinical trials;
•serious and unexpected adverse effects experienced by participants in our clinical trials;
•the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required;
•our inability to demonstrate that the clinical and other benefits of the device outweigh the risks;
•the manufacturing process or facilities we use may not meet applicable requirements; and
•the potential for approval policies or regulations of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval.
In order to sell our products in member countries of the European Economic Area (“EEA”), our products must comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC). Compliance with these requirements is a prerequisite to be able to affix the Conformité Européene (“CE”) mark to our products, without which they cannot be sold or marketed in the EEA. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can issue a European Community (“EC”) Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the EU Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a member state of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
The Company cannot be certain that it will be successful in meeting and continuing to meet the requirements to market a medical device in the EEA under the new regulatory framework called the Medical Device Regulation (“MDR”). The MDR went into force in May 2017 but allowed a three-year transition period until May 2020 for Member States, regulatory
69
authorities, and medical device stakeholders to come into compliance with the new requirements. A one-year delay of the compliance date of the MDR was implemented in response to the COVID-19 pandemic, and the directive entered into application on May 26, 2021. Compared to the earlier regulatory framework of Medical Device Directive (“MDD”), the MDR promotes a shift from the pre-approval stage (i.e., the path to CE Marking) to a life-cycle approach and places greater emphasis on clinical data and clinical evaluations to assure the safety and performance of new medical devices. Moreover, the MDR includes elements intended to strengthen the conformity assessment procedures, assert greater control over notified bodies and their standards, increase overall system transparency, and impose more robust device vigilance requirements on manufacturers and distributors. The new rules and procedures that have been created under the overhauled European regulations will likely result in increased regulatory oversight of all medical devices marketed in the European Union, and this may, in turn, increase the costs, time and requirements that need to be met in order to place an innovative or high-risk medical device on the EEA market.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. If we fail to remain in compliance with applicable European laws and directives, we would be unable to continue to affix the CE mark to our products, which would prevent us from selling them within the EEA. Approval and CE marking procedures vary between countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval or CE mark in other countries might differ from that required to obtain FDA clearance. The regulatory approval or CE marking process in other countries may include all of the risks detailed above regarding FDA clearance in the United States. Regulatory approval or the CE marking of a product in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval or a CE mark in one country may negatively impact the regulatory process in others. Failure to obtain regulatory approval or a CE mark in other countries or any delay or setback in obtaining such approval could have the same adverse effects described above regarding FDA clearance in the United States.
Recent initiatives by the FDA to enhance and modernize various regulatory pathways for device products and its overall approach to safety and innovation in the medical technology industry creates the possibility of changing product development costs, requirements, and other factors and additional uncertainty for the Company’s future products and business.
Regulatory requirements may change in the future in a way that adversely affect the Company. Any change in the laws or regulations that govern the clearance and approval processes or the post-market compliance requirements relating to the Company’s current and future products and associated services could make it more difficult and costly to obtain clearance or approval for new products, or to produce, market and distribute existing products. For example, the FDA and other government agencies have been focusing on the cybersecurity risks associated with certain medical devices and encouraging device manufacturers to take a more proactive approach to assessing the cybersecurity risks of their devices both during development and on a periodic basis after the devices are in commercial distribution. These regulatory efforts could lead to new FDA requirements in the future or additional product liability or other litigation risks, if any of the Company’s products and associated services are considered susceptible to third-party tampering.
In December 2016, Congress passed the 21st Century Cures Act, which made multiple changes to the FDA’s rules for medical devices as well as for clinical trials. In August 2017, Congress passed the Medical Device User Fee reauthorization package, which affects medical device regulation both pre- and post-approval and could have certain impacts on our business. Since that time, the FDA has announced a series of efforts to modernize and streamline the 510(k) notification and regulatory review process and monitoring post-market safety, and issued a proposed rule to formalize the de novo classification process to provide clarity to innovative device developers. Changes in the FDA 510(k) process could make clearance more difficult to obtain, increase delay, add uncertainty and have other significant adverse effects on our ability to obtain and maintain clearance for the Company’s products and associated services.
It is unclear at this time whether and how various activities initiated or announced by the FDA to modernize the U.S. medical device regulatory system could affect the Company’s business, as some of the FDA’s new medical device safety and innovation initiatives have not been formalized and remain subject to change. For example, a 2018 Medical Device Safety Action Plan announced by former FDA Commissioner Gottlieb included a particular focus on post-market surveillance and how to respond when new safety concerns emerge once a product is on the market. The increased attention that the medical technology industry is receiving from FDA leadership that understands the challenging and rapidly
70
changing nature of the U.S. health care system creates the possibility of unanticipated regulatory and other potential changes to the Company’s products and its overall business.
Failure to comply with post-marketing regulatory requirements could subject us to enforcement actions, including substantial penalties, and might require us to recall or withdraw a product from the market.
We are subject to ongoing and pervasive regulatory requirements governing, among other things, the manufacture, marketing, advertising, medical device reporting, sale, promotion, import, export, registration, and listing of devices. For example, we are required to submit periodic reports to the FDA as a condition of 510(k) clearance. These reports include information about failures and certain adverse events associated with the device after its clearance. Failure to submit such reports, or failure to submit the reports in a timely manner, could result in enforcement action by the FDA. Following its review of the periodic reports, the FDA might ask for additional information or initiate further investigation.
The regulations to which we are subject are complex and have become more stringent over time. Regulatory changes could result in restrictions on our ability to continue or expand our operations, higher than anticipated costs, or lower than anticipated sales. Even after we have obtained the proper regulatory clearance to market a device, we have ongoing responsibilities under FDA regulations and applicable foreign laws and regulations. The FDA, state and foreign regulatory authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA, state or foreign regulatory authorities, which may include any of the following sanctions:
•untitled letters or warning letters;
•fines, injunctions, consent decrees and civil penalties;
•recalls, termination of distribution, administrative detention, or seizure of our products;
•customer notifications or repair, replacement or refunds;
•operating restrictions or partial suspension or total shutdown of production;
•delays in or refusal to grant our requests for future clearances or approvals or foreign marketing authorizations of new products, new intended uses, or modifications to existing products;
•withdrawals or suspensions of product clearances or approvals, resulting in prohibitions on sales of our products;
•FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; and
•criminal prosecution.
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, financial condition and results of operations. In addition, the FDA or state or foreign authorities may change their clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay clearance or approval of our future products under development on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain new clearances or approvals, increase the costs of compliance or restrict our ability to maintain any approvals we are able to obtain.
Our products must be manufactured in accordance with federal, state and foreign regulations, and we could be forced to recall our devices or terminate production if we fail to comply with these regulations.
The methods used in, and the facilities used for, the manufacture of our products must comply with the QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of medical devices. As manufacturers of electron radiation-emitting products, we are also responsible for compliance with the radiological health regulations and certain radiation safety performance standards.
Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. Our products are also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing.
71
Our third-party manufacturers may not take the necessary steps to comply with applicable regulations, which could cause delays in the delivery of our products. In addition, failure to comply with applicable FDA or state or foreign requirements or later discovery of previously unknown problems with our products or manufacturing processes could result in, among other things: warning letters or untitled letters; fines, injunctions or civil penalties; suspension or withdrawal of approvals; seizures or recalls of our products; total or partial suspension of production or distribution; administrative or judicially imposed sanctions; the FDA’s refusal to grant pending or future clearances or approvals for our products; clinical holds; refusal to permit the import or export of our products; and criminal prosecution of us, our suppliers or our employees.
Any of these actions could significantly and negatively affect supply of our products. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we could lose customers and experience reduced sales and increased costs.
We have limited experience in identifying and working with large-scale contracts with medical device manufacturers.
To achieve the levels of production necessary to commercialize the QT Breast Scanner and any other future products or product candidates, we have entered into the Canon Manufacturing Agreement with CMSC for the manufacture of the QT Breast Scanner (and will need to something similar for other future products or product candidates), and by doing so, have secured a large-scale manufacturing agreement with a contract manufacturer that complies with the manufacturing standards prescribed by various federal, state and local regulatory agencies in the U.S. and any other country of use. We have limited experience coordinating and overseeing the manufacturing of medical device products on a large-scale. Manufacturing and control problems could arise as we attempt to commercialize our products and manufacturing may not be completed in a timely manner or at a commercially reasonable cost. In addition, we may not be able to adequately finance the manufacturing and distribution of our products on terms acceptable to us, if at all. If we cannot successfully oversee and finance the manufacturing of our products after receiving regulatory approval, we may not generate sufficient revenue to become profitable.
The misuse or off-label use of our products may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
Advertising and promotion of our existing product, and products under development that obtain approval in the United States may be heavily scrutinized by the FDA, the DOJ, HHS, state attorneys general, members of Congress, and the public. In addition, advertising and promotion of any future product that obtains approval outside of the United States will be heavily scrutinized by comparable foreign regulatory authorities.
The QT Breast Scanner is, and we expect will continue to be, cleared by the requisite regulatory authorities for specific indications. We expect to train our marketing personnel and direct sales force to not promote our devices for uses outside of the FDA-approved indications for use, known as “off-label uses.” We cannot, however, prevent a physician from using our devices off-label, when in the physician’s independent professional medical judgment, he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use our devices off-label. Furthermore, the use of our devices for indications other than those approved by the FDA or approved by any foreign regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among healthcare providers and patients.
If the FDA or any state or foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of an untitled letter, which is used for violators that do not necessitate a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations. We may become subject to such actions and, if we are not successful in defending against such actions, those actions may have a material adverse effect on our business, financial condition and results of operations. Equivalent laws and potential consequences exist in foreign jurisdictions.
In addition, if our products are cleared or approved, healthcare providers may misuse our products or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If our devices are misused or used with improper technique, we may become subject to costly litigation by our customers or their
72
patients. As described above, product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance.
Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
Our existing product and products under development that receive clearance or approval will be subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of the product. If we fail to comply with our reporting obligations, the FDA or other regulatory bodies could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device clearance or approval, seizure of our products or delay in clearance or approval of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. Product defects or other errors may occur in the future.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new clearances or approvals for the device before we may market or distribute the corrected device. Seeking such clearances or approvals may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
Our QT Breast Scanner technology may become obsolete.
Our QT Breast Scanner may become obsolete prior to commercialization by new scientific or technological developments, or by others with new technologies that are more efficient, precise and/or more economical than the QT Breast Scanner or our future product candidates. Any one of our competitors could develop a more effective product which would render our technology obsolete. In addition, it is possible that competitors may use similar technologies, equipment or devices to attempt to create a product similar to the QT Breast Scanner. Further, new technological and scientific developments could cause our QT Breast Scanner and future product candidates to become obsolete. Further developments and innovation in the area of medical imaging could require us to reconfigure the QT Breast Scanner or our future product candidates, which may not be commercially feasible, or cause them to become obsolete. Lastly, our ability to achieve significant and sustained growth in our key target markets will depend upon our success in market penetration, utilization, publication, our reimbursement efforts and medical education. Our products may not remain competitive with products based on new technologies. If we fail to sell products that satisfy our customers’ demands, or respond effectively to new
73
product announcements by our competitors, then market acceptance of our products could be reduced and our business, results of operations and financial condition could be adversely affected.
Our relationships with customers and third-party payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Physicians, other healthcare providers, and third-party payors will play a primary role with respect to our current products and any future products for which we obtain marketing approval. Our arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our product. Restrictions under applicable federal and state healthcare laws and regulations include the following:
•The U.S. federal healthcare program Anti-Kickback Statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly and practices that involve remuneration to those who prescribe, purchase, or recommend medical devices, including certain discounts, or engaging consultants as speakers or consultants, may be subject to scrutiny if they do not fit squarely within the exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability. Moreover, there are no safe harbors for many common practices, such as educational and research grants. Liability may be established without a person or entity having actual knowledge of the Anti-Kickback Statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of the Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act. Due to the breadth of these laws, the narrowness of statutory exceptions and regulatory safe harbors available, and the range of interpretations to which they are subject, it is possible that some of our current or future practices might be challenged under one or more of these laws. The Company’s compliance with Medicare and Medicaid regulations may be reviewed by federal or state agencies, including the OIG, CMS, and DOJ, or may be subject to whistleblower lawsuits under federal and state false claims laws.
•The federal civil False Claims Act prohibits, among other things, any person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds, or knowingly making, using, or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. In recent years, several healthcare companies have faced enforcement actions under the federal False Claims Act for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product or causing false claims to be submitted because of the company’s marketing the product for unapproved, and thus non-reimbursable, uses. False Claims Act liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of tens of thousands of dollars per false claim or statement. Healthcare companies also are subject to other federal false claims laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs.
•The HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), imposes criminal and civil liability for knowingly and willfully executing a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits, items or services. In addition, HIPAA, as amended by HITECH, and their respective implementing regulations impose obligations, including mandatory contractual terms, on covered healthcare providers, health plans, as well as their business associates, with respect to safeguarding the privacy, security and transmission of individually identifiable health information.
•The Physician Payment Sunshine Act, implemented as the Open Payments program, requires manufacturers of certain products reimbursed by Medicare, Medicaid, or the Children’s Health Insurance Program to track and report to the federal government payments and transfers of value that they make to physicians and teaching hospitals, certain other healthcare professionals beginning in 2022, group purchasing organizations, and
74
ownership interests held by physicians and their families, and provides for public disclosures of these data. Manufacturers are required to submit annual reports to the government and failure to do so may result in civil monetary penalties for all payments, transfers of value and ownership or investment interests not reported in an annual submission, and may result in liability under other federal laws and regulations.
•Many states have adopted laws and regulations analogous to the federal laws cited above, including state anti-kickback and false claims laws, which may apply to items or services reimbursed under Medicaid and other state programs or, in several states, regardless of the payer. Several states have enacted legislation requiring medical device companies to, among other things, establish marketing compliance programs; file periodic reports with the state, including reports on gifts and payments to individual health care providers; make periodic public disclosures on sales, marketing, pricing, clinical trials and other activities; and/or register their sales representatives. Some states prohibit specified sales and marketing practices, including the provision of gifts, meals, or other items to certain health care providers.
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations involve substantial costs. Additionally, it is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. Exclusion, suspension and debarment from government funded healthcare programs would significantly impact our ability to commercialize, sell or distribute any product. If any of the physicians or other providers or entities with whom we expect to do business are found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Changes in laws or regulations relating to data protection, or any actual or perceived failure by us to comply with such laws and regulations or our privacy policies, could materially and adversely affect our business or could lead to government enforcement actions and significant penalties against us, and adversely impact our operating results.
We may receive health information and other highly sensitive or confidential information and data of patients and other third parties, which we may compile and analyze. Collection and use of this data might raise privacy and data protection concerns, which could negatively impact our business. There are numerous federal, state and international laws and regulations regarding privacy, data protection, information security, and the collection, storing, sharing, use, processing, transfer, disclosure, and protection of personal information and other data, and the scope of such laws and regulations may change, be subject to differing interpretations, and may be inconsistent among countries and regions we intend to operate in (e.g., the U.S., the European Union and Israel), or conflict with other laws and regulations. The regulatory framework for privacy and data protection worldwide is, and is likely to remain for the foreseeable future, uncertain and complex, and this or other actual or alleged obligations may be interpreted and applied in a manner that we may not anticipate or that is inconsistent from one jurisdiction to another and may conflict with other rules or practices including ours. Further, any significant change to applicable laws, regulations, or industry practices regarding the collection, use, retention, security, or disclosure of data, or their interpretation, or any changes regarding the manner in which the consent of relevant users for the collection, use, retention, or disclosure of such data must be obtained, could increase our costs and require us to modify our services and candidate products, possibly in a material manner, which we may be unable to complete, and may limit our ability to store and process patients’ data or develop new services and features.
In particular, we will be subject to U.S. data protection laws and regulations (i.e., laws and regulations that address privacy and data security) at both the federal and state levels. The legislative and regulatory landscape for data protection continues to evolve, and in recent years there has been an increasing focus on privacy and data security issues. Numerous federal and state laws, including state data breach notification laws, state health information privacy laws, and federal and state consumer protection laws, govern the collection, use, and disclosure of health-related and other personal information. Failure to comply with such laws and regulations could result in government enforcement actions and create liability for us (including the imposition of significant civil or criminal penalties), private litigation and/or adverse publicity that could negatively affect our business. For instance, California enacted the CCPA on June 28, 2018, which took effect on January 1, 2020. The CCPA creates individual privacy rights for California consumers and increases the privacy and security obligations of entities handling certain personal data. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. The CCPA may increase our compliance costs and potential liability, and many similar laws have been proposed at the federal level and in other states.
75
In addition, we expect to obtain health information that is subject to privacy and security requirements under HITECH and its implementing regulations. The privacy standards and security standards under HIPAA establish a set of standards for the protection of individually identifiable health information by health plans, health care clearinghouses and certain health care providers, (collectively referred to as “Covered Entities”), and the business associates with whom Covered Entities enter into service relationships pursuant to which individually identifiable health information may be exchanged. Notably, whereas HIPAA previously directly regulated only Covered Entities, HITECH makes certain of HIPAA’s privacy and security standards also directly applicable to Covered Entities’ business associates. As a result, both Covered Entities and business associates are now subject to significant civil and criminal penalties for failure to comply with privacy standards and security standards. As part of our normal operations, we expect to collect, process and retain personal identifying information regarding patients, including as a business associate of Covered Entities, so we expect to be subject to HIPAA, including changes implemented through HITECH, and we could be subject to criminal penalties if we knowingly obtain or disclose individually identifiable health information in a manner that is not authorized or permitted by HIPAA. A data breach affecting sensitive personal information, including health information, also could result in significant legal and financial exposure and reputational damages that could potentially have an adverse effect on our business.
HIPAA requires Covered Entities (like many of our potential customers) and business associates, like us, to develop and maintain policies and procedures with respect to protected health information that is used or disclosed, including the adoption of administrative, physical and technical safeguards to protect such information. HITECH expands the notification requirement for breaches of patient-identifiable health information, restricts certain disclosures and sales of patient-identifiable health information and provides for civil monetary penalties for HIPAA violations. HITECH also increased the civil and criminal penalties that may be imposed against Covered Entities and business associates and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce HIPAA and its implementing regulations and seek attorney’s fees and costs associated with pursuing federal civil actions. Additionally, certain states have adopted comparable privacy and security laws and regulations, some of which may be more stringent than HIPAA.
Internationally, many jurisdictions have or are considering enacting privacy or data protection laws or regulations relating to the collection, use, storage, transfer, disclosure and/or other processing of personal data, as well as certification requirements for the hosting of health data specifically. Such laws and regulations may include data hosting, data residency or data localization requirements (which generally require that certain types of data collected within a certain country be stored and processed within that country), data export restrictions, international transfer laws (which prohibit or impose conditions upon the transfer of such data from one country to another), or may require companies to implement privacy or data protection and security policies, enable users to access, correct and delete personal data stored or maintained by such companies, inform individuals of security breaches that affect their personal data or obtain individuals’ consent to use their personal data. For example, European legislators adopted the GDPR, which became effective on May 25, 2018, and are now in the process of finalizing the ePrivacy Regulation to replace the European ePrivacy Directive (Directive 2002/58/EC as amended by Directive 2009/136/EC). The GDPR, supplemented by national laws and further implemented through binding guidance from the European Data Protection Board, imposes more stringent European Union data protection requirements and provides for significant penalties for noncompliance. Further, the United Kingdom’s initiating a process to leave the European Union has created uncertainty with regard to the regulation of data protection in the United Kingdom. In particular, the United Kingdom has brought the GDPR into domestic law with the Data Protection Act of 2018 which will remain in force, even if and when the United Kingdom leaves the European Union.
Virtually every jurisdiction in which we expect to operate has established its own data security and privacy legal framework with which we must, and our target customers will need to, comply, including the rules and regulation mentioned above. We may also need to comply with varying and possibly conflicting privacy laws and regulations in other jurisdictions. As a result, we could face regulatory actions, including significant fines or penalties, adverse publicity and possible loss of business.
In addition, there are ongoing public policy discussions regarding whether the standards for de-identified, anonymous or pseudonymized health information are sufficient to adequately protect patient privacy. These discussions may lead to further restrictions on the use or disclosure of such information. We use a third party service provider to de-identify PHI that parties with which we work receive and may make available to us. There can be no assurance that the policy initiatives or future initiatives will not adversely affect our ability to access and use data or to develop or market current or future services, or that the third party service providers that we use to de-identify PHI will do so in a manner that is deemed compliant with any regulatory standards that currently exist or are developed in the future. There is also a risk that the third parties that license us data and enable us to receive de-identified PHI may fail to properly de-identify PHI under HIPAA or personal data under applicable state or other privacy laws, some of which may impose different standards for de-
76
identification than those required by HIPAA. Furthermore, if we are unable to secure these rights to de-identified information or because of any future changes to HIPAA or other applicable laws, we may face limitations on the use of PHI and our ability to use de-identified information that could negatively affect the scope of our product and service offering as well as impair our ability to provide upgrades and enhancements to our services.
While we are preparing to implement various measures intended to enable us to comply with applicable privacy or data protection laws, regulations and contractual obligations, these measures may not always be effective and do not guarantee compliance. Any failure or perceived failure by us to comply with our contractual or legal obligations or regulatory requirements relating to privacy, data protection, or information security may result in governmental investigations or enforcement actions, litigation, claims, or public statements against us by consumer advocacy groups or others and could result in significant liability, cause our customers, partners or patients to lose trust in us, and otherwise materially and adversely affect our reputation and business. Furthermore, the costs of compliance with, and other burdens imposed by, the laws, regulations, and policies that are applicable to the businesses of our customers or partners may limit the adoption and use of, and reduce the overall demand for, our products and services. Additionally, if third parties we work with violate applicable laws, regulations, or agreements, such violations may put the data we have received at risk, could result in governmental investigations or enforcement actions, fines, litigation, claims, or public statements against us by consumer advocacy groups or others and could result in significant liability, cause our customers, partners or patients to lose trust in us, and otherwise materially and adversely affect our reputation and business. Further, public scrutiny of, or complaints about, technology companies or their data handling or data protection practices, even if unrelated to our business, industry or operations, may lead to increased scrutiny of technology companies, including us, and may cause government agencies to enact additional regulatory requirements, or to modify their enforcement or investigation activities, which may increase our costs and risks.
Any restrictions on our ability to obtain or use data could harm our business.
Our business processes personal data, including some data related to health. When conducting clinical trials, we face risks associated with collecting trial participants’ data, especially health data, in a manner consistent with applicable laws and regulations. Any errors or defects in any third-party data or other technology could result in errors in our existing and future solutions that could harm our business and damage our reputation and cause losses in revenue, and we could be required to spend significant amounts of additional resources to fix any problems.
We may also face headwinds with limitations on the use of data in current customer contracts. We are currently evaluating those limitations and may need to renegotiate current contracts and negotiate future contracts to allow broader use of data to launch this initiative. Also, healthcare regulations concerning personal health information, including but not limited to HIPAA, HITECH, 42 CFR Part II, and their State law equivalents such as the California Consumer Privacy Act (the “CCPA”), as recently amended and expanded by the California Privacy Rights Act (the “CPRA”), could have a significant effect on the manner in which we must handle healthcare related data, and the costs of complying with such standards could be significant.
If we do not obtain and maintain international regulatory registrations, clearances or approvals for our products, we will be unable to market and sell our products outside of the United States.
Sales of our products outside of the United States are subject to foreign regulatory requirements that vary widely from country to country. Approval procedures vary among countries and can involve additional testing. The time required to obtain approval outside of the United States may differ substantially from that required to obtain FDA approval. In addition, the FDA regulates exports of medical devices from the United States. While the regulations of some countries may not impose barriers to marketing and selling our products or only require notification, others require that we obtain the clearance or approval of a specified regulatory body. Complying with foreign regulatory requirements, including obtaining registrations, clearances or approvals, can be expensive and time-consuming, and we may not receive regulatory clearances or approvals in each country in which we plan to market our products or we may be unable to do so on a timely basis. The time required to obtain registrations, clearances or approvals, if required by other countries, may be longer than that required for FDA clearance or approval, and requirements for such registrations, clearances or approvals may significantly differ from FDA requirements. If we modify our products, we may need to apply for additional regulatory clearances or approvals before we are permitted to sell the modified product. In addition, we may not continue to meet the quality and safety standards required to maintain the authorizations that we have received. If we are unable to maintain our authorizations in a particular country, we will no longer be able to sell the applicable product in that country.
Regulatory clearance or approval by the FDA does not ensure registration, clearance or approval by regulatory authorities in other countries, and registration, clearance or approval by one or more foreign regulatory authorities does not ensure registration, clearance or approval by regulatory authorities in other foreign countries or by the FDA. However, a
77
failure or delay in obtaining registration or regulatory clearance or approval in one country may have a negative effect on the regulatory process in others.
Legislative or regulatory reforms in the United States or the EU may make it more difficult and costly for us to obtain regulatory clearances or approvals for our products or to manufacture, market or distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulation of medical devices. In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. Over the last several years, the FDA has proposed reforms to its 510(k) clearance process, and such proposals could include increased requirements for clinical data and a longer review period, or could make it more difficult for manufacturers to utilize the 510(k) clearance process for their products. For example, in November 2018, FDA officials announced forthcoming steps that the FDA intends to take to modernize the premarket notification pathway under Section 510(k) of the FDCA. Among other things, the FDA announced that it planned to develop proposals to drive manufacturers utilizing the 510(k) pathway toward the use of newer predicates. These proposals included plans to potentially sunset certain older devices that were used as predicates under the 510(k) clearance pathway, and to potentially publish a list of devices that have been cleared on the basis of demonstrated substantial equivalence to predicate devices that are more than 10 years old. In May 2019, the FDA solicited public feedback on these proposals. The FDA requested public feedback on whether it should consider certain actions that might require new authority, such as whether to sunset certain older devices that were used as predicates under the 510(k) clearance pathway. These proposals have not yet been finalized or adopted, and the FDA may work with Congress to implement such proposals through legislation. Accordingly, it is unclear the extent to which any proposals, if adopted, could impose additional regulatory requirements on us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance, or restrict our ability to maintain our current clearances, or otherwise create competition that may negatively affect our business.
More recently, in September 2019, the FDA finalized guidance describing an optional “safety and performance based” premarket review pathway for manufacturers of “certain, well-understood device types” to demonstrate substantial equivalence under the 510(k) clearance pathway by showing that such device meets objective safety and performance criteria established by the FDA, thereby obviating the need for manufacturers to compare the safety and performance of their medical devices to specific predicate devices in the clearance process. The FDA intends to develop and maintain a list device types appropriate for the “safety and performance based” pathway and will continue to develop product-specific guidance documents that identify the performance criteria for each such device type, as well as the testing methods recommended in the guidance documents, where feasible. The FDA may establish performance criteria for classes of devices for which we or our competitors seek or currently have received clearance, and it is unclear the extent to which such performance standards, if established, could impact our ability to obtain new 510(k) clearances or otherwise create competition that may negatively affect our business.
In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain clearance or approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require: additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be promulgated that could prevent, limit or delay regulatory clearance or approval of our future products. We cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If we are slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if we are not able to maintain regulatory compliance, we may lose any marketing approval or clearance that we may have obtained and we may not achieve or sustain profitability.
On April 5, 2017, the European Parliament passed the MDR (Regulation 2017/745), which repeals and replaces the EU Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the European Economic Area (EEA) Member States, the regulations would be directly applicable, i.e., without the need for adoption of EEA member state laws implementing them, in all EEA Member States and are intended to eliminate current differences in the regulation of medical devices among EEA Member States. The MDR, among other things, is intended to establish a
78
uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation.
The MDR become applicable three years after publication (in 2020). The new regulations will, among other things:
•strengthen the rules on placing devices on the market and reinforce surveillance once they are available;
•establish explicit provisions on manufacturers’ responsibilities for follow-up regarding the quality, performance and safety of devices placed on the market;
•improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number;
•set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; and
•strengthened rules for the assessment of certain high-risk devices, which may have to undergo an additional check by experts before they are placed on the market.
These modifications may have an effect on the way we conduct our business in the EEA.
Healthcare reform laws could adversely affect our products and financial condition.
During the past several years, the U.S. healthcare industry has been subject to an increase in governmental regulation at both the federal and state levels. Efforts to control healthcare costs, including limiting access to care, alternative delivery models and changes in the methods used to determine reimbursement scenarios and rates, are ongoing at the federal and state government levels.
In March 2010, former President Obama signed into law the ACA, which included measures that significantly changed the way healthcare is financed by both governmental and private insurers. While a primary goal of these healthcare reform efforts was to expand coverage to more individuals, it also involved additional regulatory mandates and other measures designed to constrain medical costs. The ACA significantly impacts the medical device industry. Among other things, the ACA:
•imposes an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States, which, through a series of legislative amendments, was suspended, effective January 1, 2016 and subsequently repealed altogether on December 20, 2019;
•establishes a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; and
•implements Medicare payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models.
In addition, the ACA and related healthcare reform laws, regulations and initiatives have significantly increased regulation of managed care plans and decreased reimbursement under Medicare managed care. Moreover, to alleviate budget shortfalls, states have reduced or frozen payments to Medicaid managed care plans. We cannot accurately predict the complete impact of these healthcare reform initiatives, but they could lead to a decreased demand for medical devices and other outcomes that could adversely impact our business and financial results.
Some of the provisions of the ACA have yet to be fully implemented, and certain provisions have been subject to judicial and congressional challenges. In addition, there have been efforts by the Trump administration to repeal or replace certain aspects of the ACA and to alter the implementation of the ACA and related laws. For example, the TCJA enacted on December 22, 2017, eliminated the shared responsibility payment for individuals who fail to maintain minimum essential coverage under Section 5000A of the Internal Revenue Code of 1986, commonly referred to as the “individual mandate,” effective January 1, 2019. On December 14, 2018, a U.S. District Court Judge in the Northern District of Texas, or the Texas District Court Judge, ruled that the individual mandate is a critical and inseverable feature of the ACA, and therefore, because it was repealed as part of the Tax Cuts and Jobs Act, the remaining provisions of the ACA are invalid as well. This decision was subsequently appealed, and on December 18, 2019, the U.S. Court of Appeals for the Fifth Circuit affirmed the decision of the district court that the individual mandate, as amended by the TCJA, was unconstitutional. The Fifth Circuit remanded the case to the district court to consider a remedy, including to consider and explain which
79
provisions of the ACA are inseverable and invalid. It is unclear how this litigation, including all future hearings and appeals, and other efforts to challenge, repeal or replace the ACA, or portions thereof, will affect our future products or our business. It is possible that the ACA, as currently enacted or as it may be amended in the future, and other healthcare reform measures that may be adopted in the future, could have an adverse effect on our industry generally and on our ability to commercialize our future products and achieve profitability.
Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, cleared or approved or commercialized in a timely manner or at all, which could negatively impact our business.
The ability of the FDA to review and clear or approve new products can be affected by a variety of factors, including government budget and funding levels, statutory, regulatory, and policy changes, the FDA’s ability to hire and retain key personnel and accept the payment of user fees, and other events that may otherwise affect the FDA’s ability to perform routine functions. Average review times at the FDA have fluctuated in recent years as a result. In addition, government funding of other government agencies that fund research and development activities is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new medical devices or modifications to cleared or approved medical devices to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years, including for 35 days beginning on December 22, 2018, the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA employees and stop critical activities.
Separately, in response to the COVID-19 pandemic, on March 10, 2020 the FDA announced its intention to postpone most inspections of foreign manufacturing facilities, and on March 18, 2020, the FDA temporarily postponed routine surveillance inspections of domestic manufacturing facilities. Subsequently, on July 10, 2020 the FDA announced its intention to resume certain on-site inspections of domestic manufacturing facilities subject to a risk-based prioritization system. The FDA intends to use this risk-based assessment system to identify the categories of regulatory activity that can occur within a given geographic area, ranging from mission critical inspections to resumption of all regulatory activities. Regulatory authorities outside the United States may adopt similar restrictions or other policy measures in response to the COVID-19 pandemic. If a prolonged government shutdown occurs, or if global health concerns continue to prevent the FDA or other regulatory authorities from conducting their regular inspections, reviews, or other regulatory activities, it could significantly impact the ability of the FDA or other regulatory authorities to timely review and process our regulatory submissions, which could have a material adverse effect on our business.
Consolidation in the U.S. healthcare industry may negatively impact our results of operations.
In recent years, U.S. healthcare industry participants, including distributors, manufacturers, suppliers, healthcare providers, insurers and pharmacy chains, have consolidated or formed strategic alliances. Consolidations create larger enterprises with greater negotiating power, and also could result in the possible loss of a customer where the combined enterprise selects one distributor from two incumbents. If this consolidation trend continues, it could adversely affect our results of operations.
Failure to comply with anti-bribery and anti-corruption laws could subject us to penalties and other adverse consequences.
Since we may operate and sell our products around the world, we will be subject to the United States Foreign Corrupt Practices Act (the “FCPA”), the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the United States Travel Act, and other anti-corruption and anti-bribery laws and regulations in the jurisdictions in which we currently or may do business, both domestic and abroad. These laws and regulations generally prohibit improper payments or offers of improper payments to government officials, political parties, or commercial partners for the purpose of obtaining or retaining business or securing an improper business advantage.
Corruption issues pose a risk in every country and jurisdiction, but in many countries, particularly in countries with developing economies, it may be more common for businesses to engage in practices that are prohibited by the FCPA or other applicable laws and regulations, and our activities in these countries pose a heightened risk of unauthorized payments or offers of payments by one of our employees or third-party business partners, representatives, and agents that could be in violation of various laws including the FCPA. The FCPA and other applicable anti-bribery and anti-corruption laws also may hold us liable for acts of corruption and bribery committed by our third-party business partners, representatives, and
80
agents. We and our third-party business partners, representatives, and agents may have direct or indirect interactions with officials and employees of government agencies, or state-owned or affiliated entities and we may be held liable for the corrupt or other illegal activities of our employees or such third parties even if we do not explicitly authorize such activities. The FCPA or other applicable laws and regulations also require that we keep accurate books and records and maintain internal controls and compliance procedures designed to prevent improper payments. While we have implemented policies and procedures to address compliance with such laws, we cannot assure you that our employees or other third parties working on our behalf will not engage in conduct in violation of our policies or applicable law for which we might ultimately be held responsible. Violations of the FCPA and other applicable anti-corruption laws may result in whistleblower complaints, adverse media coverage, investigations, imposition of significant legal fees, loss of export privileges, as well as severe criminal or civil sanctions, including suspension or debarment from U.S. government contracting, and we may be subject to other liabilities and adverse effects on our reputation, which could negatively affect our business, results of operations, financial condition, and growth prospects. In addition, responding to any enforcement action may result in a significant diversion of management’s attention and resources and significant legal defense costs and other professional fees. Our exposure for violating these laws increases as our non-U.S. presence expands and as we increase sales and operations in foreign jurisdictions.
Changes in accounting principles or their application to us could result in unfavorable accounting charges or effects, which could adversely affect our results of operations and growth prospects.
We prepare our consolidated financial statements in accordance with accounting principles generally accepted in the United States (“GAAP”). In particular, we make certain estimates and assumptions related to the adoption and interpretation of these principles including the recognition of our revenue and the accounting of our stock-based compensation expense with respect to our consolidated financial statements. If these assumptions turn out to be incorrect, our financial results could materially differ from our expectations and could be materially adversely affected. A change in any of these principles or guidance, or in their interpretations or application to us, may have a significant effect on our reported results, as well as our processes and related controls, and may retroactively affect previously reported results or our forecasts, which may negatively impact our consolidated financial statements.
If our judgments or estimates relating to our critical accounting policies are based on assumptions that change or prove to be incorrect, our results of operations could fall below expectations of securities analysts and investors, resulting in a decline in our stock price.
The preparation of our consolidated financial statements in conformity with GAAP requires management to make judgments, estimates, and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, as provided in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” the results of which form the basis for making judgments about the carrying values of assets, liabilities, and equity, and the amount of revenue and expenses that are not readily apparent from other sources. Our results of operations may be adversely affected if our assumptions change or if actual circumstances differ from those in our assumptions, which could cause our results of operations to fall below the expectations of securities analysts and investors, resulting in a decline in the trading price of our common stock. Significant judgments, estimates, and assumptions used in preparing our consolidated financial statements include, or may in the future include, those related to revenue recognition, stock-based compensation, intangible assets, including goodwill, and income taxes.
We could be subject to additional tax liabilities.
We are subject to federal, state, and local income taxes in the US. Determining our provision for income taxes requires significant management judgment, and the ultimate tax outcome may be uncertain. In addition, our provision for income taxes is subject to volatility and could be adversely affected by many factors, including, among other things, changes to our operating or holding structure, changes in the amounts of earnings in jurisdictions with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities, and changes in U.S. tax laws. Tax authorities may disagree with our calculation of research and development tax credits, cross-jurisdictional transfer pricing, or other matters and assess additional taxes, interest, or penalties. While we regularly assess the likely outcomes of these examinations to determine the adequacy of our provision for income taxes and we believe that our financial statements reflect adequate reserves to cover any such contingencies, there can be no assurance that the outcomes of such examinations will not have a material impact on our results of operations and cash flows. If tax authorities change applicable tax laws, our overall taxes could increase, and our financial condition or results of operations may be adversely impacted.
81
Risks Related to our Intellectual Property
It is difficult and costly to protect our intellectual property and our proprietary technologies, and we may not be able to ensure their protection.
We rely upon a combination of patents and trade secrets to protect the intellectual property related to our proprietary technologies. Our success depends significantly on our ability to obtain and maintain intellectual property protection with respect to our technology and products. Patents and other proprietary rights provide uncertain protections, and we may be unable to protect our intellectual property for reasons including those that result from complex factual and legal issues such as those that create uncertainty as to the validity, scope and enforceability of any particular patent that we hold or for which we have applied. As a result, we may be unsuccessful in defending our patents and other proprietary rights against third-party challenges, which could have a material adverse effect on our business.
Our company uses a combination of patents, trademarks and copyrights to protect our intellectual property. Although we currently have active U.S. and European patents and patents pending with the U.S. Patent & Trademark Office and have filed to obtain patent coverage for our technology in the UK, France, Germany, Italy, Netherlands and Spain, there are aspects of the technology for which patent coverage may never be sought or received. Additionally, we may in the future obtain, certain intellectual property related to our technology from third parties, and we cannot be certain that such third parties took the necessary actions to maintain such rights or that the transfer of such rights to us was proper and effective. We may, as a result, be subject to claims challenging the ownership or enforceability of such rights. Furthermore, we may not possess the resources to, or for other reasons may not choose to, pursue patent protection on every invention or in any or every country where we may eventually decide to sell our future products. Our ability to prevent others from making or selling duplicate or similar technologies will be impaired for those technologies with respect to which, and in those countries where, we have no patent protection. In addition, there is no assurance that all potentially relevant prior art relating to our patents and patent applications has been found, which can prevent a patent from issuing from a pending patent application or later invalidate or narrow the scope of an issued patent. Even if patents do successfully issue and even if such patents cover our technology, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed, invalidated, or held unenforceable. Any successful challenge to these patents or any other patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of our technology.
In addition, for patents that do issue based on our applications or future applications, any issued patents may not provide us with any competitive advantages. Competitors may be able to design around our patents and develop products that provide outcomes comparable or superior to ours. Any changes we make to our product or any future products, including designs that may be required for commercialization or that cause them to have what we view as more advantageous properties, may not be covered by patents and patent applications we have licensed or own, and we may be required to file new applications and/or seek other forms of protection for any such altered products if any such protection is available. In addition, the patent prosecution process is expensive, time-consuming and complicated, and we and our current or future licensors, licensees or collaborators may not be able to prepare, file, prosecute and maintain all necessary or desirable patents or patent applications at a reasonable cost or in a timely manner. It is also possible that we or our current or future licensors, licensees or collaborators will fail to identify patentable aspects of inventions before it is too late to obtain patent protection for them. In addition, if we choose to and are able to secure patent protection in countries outside the U.S., the laws of some foreign countries may not protect our intellectual property rights to the same extent as do the laws of the United States. For instance, the legal systems of some countries, including India, China and other developing countries, do not favor the enforcement of patents and other intellectual property rights. This could make it difficult for us to stop the infringement of our patents or the misappropriation of our other intellectual property rights.
Some countries also have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, some countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired.
Changes in either the patent laws or their interpretation in the United States and other countries may diminish our ability to protect our inventions and enforce our intellectual property rights, and more generally could affect the value of our intellectual property. Our efforts to seek patent protection for our technology could be negatively impacted by any such changes, which could have a material adverse effect on our existing patent rights and our ability to protect and enforce our intellectual property in the future. In particular, our ability to stop third parties from making, using, selling, offering to sell or importing products that infringe our intellectual property will depend in part on our success in obtaining and enforcing patent claims that cover our technology, inventions and improvements.
82
We may come to believe that third parties are infringing on, or otherwise violating, our patents or other proprietary rights. To prevent infringement or unauthorized use, we may need to file infringement and/or misappropriation suits, which are very expensive and time-consuming, could result in meritorious counterclaims against us and would distract management’s attention. Also, in an infringement or misappropriation proceeding, a court may decide that one or more of our patents is invalid, unenforceable, or both, in which case third parties may be able to use our technology without paying license fees or royalties. Even if the validity of our patents is upheld, a court may refuse to stop the other party from using the technology at issue on the grounds that the other party’s activities are not covered by our patents.
In addition to patents, we rely on trade secrets to protect our technology; however, the policies we use to protect our trade secrets may not be effective in preventing misappropriation of our trade secrets by others. In addition, confidentiality agreements executed by our employees, consultants and advisors may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure. Litigating a trade secret claim is expensive and time consuming, and the outcome may be unexpected. In addition, courts outside the United States are sometimes less willing to protect trade secrets. Moreover, our competitors may independently develop knowledge, methods and know-how that allow them to create substantially similar products or services without misappropriating our trade secrets. If we are unable to protect our trade secrets, we may be unable to prevent competitors from using our own inventions and intellectual property to compete against us, and our business may be harmed.
If we are unable to protect or enforce our patents or other proprietary rights, or if we become subject to claims or litigation alleging infringement of the patents or other proprietary rights of others, our competitiveness and business prospects may be materially damaged.
Patent and other proprietary rights are essential to our business. Our success depends to a significant degree on our ability to obtain and enforce patents and licenses to patent rights, both in the United States and in other countries. We cannot guarantee that our pending patent applications, or any future patent applications, will result in issued patents, our patents issued or licensed will not be challenged or circumvented by competitors, our patents will not be found to be invalid or the intellectual property rights of others will not prevent us from selling certain products or including key features in our products.
The patent position of a healthcare company is often uncertain and involves complex legal and factual questions. Significant litigation concerning patents and products is pervasive in our industry. Patent claims include challenges to the coverage and validity of our patents on products or processes as well as allegations that our products infringe patents held by competitors or other third parties. An unfavorable litigation outcome in any of these types of cases could result in a loss of patent protection or the ability to market products, which could lead to a significant loss of sales, or otherwise materially affect our business, results of operations, financial condition and cash flows. We also rely on trademarks, copyrights, trade secrets and know-how to develop, maintain and strengthen our competitive positions. Third parties may know, discover or independently develop equivalent proprietary information or techniques, or they may gain access to our trade secrets or publicly disclose our trade secrets.
Although our employees, consultants, parties to collaboration agreements and other business partners are generally subject to confidentiality or similar agreements to protect our confidential and proprietary information, these agreements may be breached, and we may not have adequate remedies for any breach. To the extent that our employees, consultants, parties to collaboration agreements and other business partners use intellectual property owned by others in their work for us, disputes may arise as to the rights in related or resulting know-how and inventions.
Furthermore, our intellectual property, proprietary technology and sensitive company data is potentially vulnerable to loss, damage and misappropriation from system malfunction, computer viruses and unauthorized access to our data or misappropriation or misuse thereof by those with permitted access and other events. While we have invested to protect our intellectual property, confidential information and other data, and continue to work diligently in this area, there can be no assurance that our precautionary measures have prevented or will prevent future breakdowns, breaches, cyber incidents or other events. Any of the events referenced above could have a material adverse effect on our reputation, business, results of operations, financial condition and cash flows.
Patent terms may be inadequate to protect our competitive position on our future products for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our future products are obtained, once the patent life has expired, we may be open to competition from competitive products.
83
Given the amount of time required for the development, testing and regulatory review of new products, patents protecting our future products might expire before or shortly after we or our future partners commercialize those products. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours for a sufficient amount of time, and, as a result, we may not be able to obtain adequate protection from our patent portfolio against competition, in spite of the time and effort invested in the commercialization of our future products.
Claims that our technology or our future products or the sale or use of our future products infringe the patents or other intellectual property rights of third parties could result in costly litigation or could require substantial time and money to resolve, even if litigation is avoided.
Because our industry is characterized by competing intellectual property, we may be subject to legal actions for violating the intellectual property rights of others, including claims that former employees, collaborators or third parties have an interest in our patents, trade secrets or other intellectual property. For example, we may have inventorship or ownership disputes arising from conflicting obligations of employees, consultants or others who are involved in developing our technology or our products.
We also may be required to participate in interference, derivation or opposition proceedings that concern disputes regarding priority of inventions disclosed in our patents. Determining whether a product infringes a patent, as well as priority of inventions and other patent-related disputes, involves complex legal and factual issues and the outcome is often uncertain. We have not conducted any significant search of patents issued to third parties, and third-party patents containing claims covering our technology or methods that predate our patents may exist. Because of the number of patents issued and patent applications filed in our technical areas or fields (including some pertaining specifically to medical imaging technologies), our competitors or other third parties may assert that our technology and the methods we employ in the use of products incorporating our technology are covered by United States or foreign patents held by them. In addition, because patent applications can take many years to issue and because publication schedules for pending applications vary by jurisdiction, there may be applications now pending of which we are unaware, and which may result in issued patents that our technology or other future products would infringe. Also, because the claims of published patent applications can change between publication and patent grant, there may be published patent applications that may ultimately issue with claims that we infringe.
As the number of competitors in the market for medical imaging technologies increases, and as the number of patents issued in this area grows, the possibility of patent infringement claims against us increases. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can, including if they have substantially greater resources. Defending against such litigation is costly and time consuming, and would distract our management from our business. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations.
In the event that we become subject to a patent infringement or other intellectual property lawsuit and if the relevant patents or other intellectual property were upheld as valid and enforceable and we were found to infringe or violate those rights or the terms of a license to which we are a party, we could be prevented from selling any infringing products of ours unless we could obtain a license or were able to redesign the product to avoid infringement. If we were unable to obtain a license or successfully redesign, we might be prevented from selling our technology or other future products. If we are able to redesign, we may need to invest substantial resources in the redesign process. If there is an allegation or determination that we have infringed the intellectual property rights of a competitor or other person, we may be required to pay damages, or a settlement or ongoing royalties, or we may be required to enter into cross-licenses with our competitors. In any of these circumstances, we may be unable to sell our products at competitive prices or at all, and our business, financial condition, results of operations and prospects could be harmed.
In addition, we may be required to indemnify our customers and distributors against claims relating to the infringement of intellectual property rights of third parties related to our products. Third parties may assert infringement claims against our customers or distributors. These claims may require us to initiate or defend protracted and costly litigation on behalf of our customers or distributors, regardless of the merits of these claims. If any of these claims succeed, we may be forced to pay damages on behalf of our customers or distributors, or may be required to obtain licenses for the products or services they use. If we cannot obtain all necessary licenses on commercially reasonable terms, our distributors may be forced to stop distributing our products or services, and our customers may be forced to stop using our products or services.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during discovery. There could also be public announcements of the results of hearings, motions or other interim proceedings or
84
developments, which could have a material adverse effect on the price of our common stock. If securities analysts or investors perceive these results to be negative, it could have a material adverse effect on the price of our common stock.
Obtaining and maintaining patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated if we or our future licensors do not comply with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other government fees on a patent and patent application are due to be paid to the patent offices and agencies in several stages over the lifetime of the patent and patent application. The U.S. Patent and Trademark Office and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. In certain circumstances, we may be required to rely on our licensing partners to take the necessary action to comply with these requirements with respect to patents or other intellectual property they have licensed to us. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance, which could include failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents, can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, our competitors may be able to enter the market and compete with our products, which would have a material adverse effect on our business.
We may be subject to claims that our employees, consultants or advisers have wrongfully used or disclosed alleged trade secrets of their former employers or claims asserting ownership of what we regard as our own intellectual property.
Many of our employees, consultants and advisers, including our senior management, were previously employed at other companies that may have proprietary rights related to our business. Some of these employees, consultants and advisers, including members of our senior management, executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we try to ensure that such individuals do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these employees have used or disclosed intellectual property, including trade secrets or other proprietary information, of any such individual’s former employer. We are not aware of any such disclosures, or threatened or pending claims related to these matters, but in the future, litigation may be necessary to defend against such claims. If we fail in defending any such claims, we may lose valuable intellectual property rights or personnel, in addition to possibly paying monetary damages and being enjoined from conducting our business as contemplated. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management.
Additionally, a licensor, collaborator, employee, consultant, adviser or other third party may dispute our or our licensor’s ownership of certain intellectual property rights. We seek to address these concerns in our contractual agreements; however, we may not have contractual arrangements with the party in question and/or such provisions may not be effective. If these provisions prove to be ineffective, we may not be able to achieve our business objectives. If we or our licensors fail in defending any such claims, we may have to pay monetary damages and may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, intellectual property, which could adversely impact our business, financial condition and results of operations.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered and unregistered trademarks or trade names are valuable assets and may be challenged, infringed, circumvented or declared generic or determined to infringe third party’s marks. We may not be able to protect our rights to these trademarks and trade names, which may be necessary to build name recognition among potential collaborators or customers in our markets of interest. At times, competitors may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other trademarks or trademarks that incorporate variations of our unregistered trademarks or trade names. We have not conducted any registrability studies for possible future trademarks to assess whether such marks would be successfully registered. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. In addition, we may license our trademarks and trade names to third parties, such as distributors. Though these license agreements may provide guidelines for how our trademarks and trade names may be used, a breach of these agreements or misuse of our trademarks and tradenames by our licensees may jeopardize our rights in or diminish the goodwill associated with our trademarks and trade names. Our efforts to enforce or protect our proprietary rights related to trademarks, trade names, trade secrets, domain names, copyrights or other intellectual property
85
may be ineffective and could result in substantial costs and diversion of resources and adversely affect our competitive position, business, financial condition, results of operations and prospects.
Our rights to develop and commercialize our products may be subject to the terms and conditions of licenses and sublicenses granted to us by third parties.
We rely on licenses and sublicenses to certain patent rights and other intellectual property from third parties that are important or necessary to the development of our products, including the software modules and cloud software that are integrated into the QT Breast Scanner and products and services. These and other licenses may not provide exclusive rights to use such intellectual property in all relevant fields of use and in all territories in which we may wish to develop or commercialize our products and the underlying patents may fail to provide the intended exclusivity. As a result, we may not be able to prevent competitors from developing and commercializing competitive products in the markets that we hope to address. Moreover, we would not own at least some of the underlying intellectual property rights related to these products, and as a result our rights would be subject to the continuation and compliance with the terms of those agreements. If such in-licenses were terminated, competitors would have the freedom to develop, seek regulatory approval of, and to market, products similar or identical to ours.
In addition, these license agreements may not grant us the right to control the preparation, filing, prosecution or maintenance of patents and patent applications covering our products. Therefore, we cannot be certain that these patents and patent applications will be prepared, filed, prosecuted or maintained in a manner consistent with the best interests of our business. If our current or future licensing partners fail to file, prosecute or maintain such patents, including the payment of applicable fees, or otherwise lose rights to those patents or patent applications, the intellectual property we have licensed or exclusivity we have been granted may be reduced or eliminated, and our right to develop and commercialize any of our future products that are subject of such licensed rights, and our ability to prevent competitors from developing or commercializing such products, could be adversely affected. In addition, even where we have the right to control patent prosecution and maintenance of patents and patent applications we have licensed from third parties, we may still be adversely affected or prejudiced by actions or inactions of our licensees, our licensors and their counsel that took place prior to the date upon which we assumed control over patent prosecution.
Pursuant to the terms of such license agreements, the licensors may also have the right to control enforcement of our licensed patents or defense of any claims asserting the invalidity or unenforceability of these patents. Even if we are permitted to pursue the enforcement or defense of our licensed patents, we may require the cooperation of our future licensors or collaboration partners and any other applicable patent owners and we cannot be certain that such cooperation will be provided to us. We also cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents. Even if we are not a party to these legal actions, an adverse outcome could harm our business because it might prevent us from continuing to license intellectual property that we may need to operate our business. If we lose any of our licensed intellectual property, our right to develop and commercialize any of our products that are subject of such licensed rights could be adversely affected.
In addition, our future licensors may rely on third-party consultants or collaborators or on funds from third parties such that our licensors are not the sole and exclusive owners of the patents we in-license. If other third parties have ownership rights to our in-licensed patents, they may be able to license such patents to our competitors, and our competitors could market competing products and technologies. In addition, if our licensors have not obtained adequate rights from these third parties, we may need to obtain additional rights from these third parties or we could be prevented from developing and commercializing the related products. This could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
In spite of our best efforts, our licensors might conclude that we have materially breached our license agreements and might therefore terminate the license agreements, in which event we may have to cease developing, manufacturing or marketing any product covered by these agreements and we may face other additional penalties or be required to grant our licensors additional rights. In addition, we may seek to obtain additional licenses from our licensors and, in connection with obtaining such licenses, we may agree to amend our existing licenses in a manner that may be more favorable to the licensors, including by agreeing to terms that could enable third parties (potentially including our competitors) to receive licenses to a portion of the intellectual property that is subject to our existing licenses. Any of these events could have a material adverse effect on our competitive position, business, financial conditions, results of operations and prospects.
86
We may be required to pay certain milestones and royalties and fulfill other obligations under our license agreements with third-party licensors.
We may be required to pay milestones and royalties related to our development or commercialization activities of our products utilizing the technologies licensed or sublicensed from third parties under license agreements we may enter into with them. These payments could adversely affect our overall profitability related to any future products that we may seek to develop or commercialize. In order to maintain our license rights under our license agreements, we may need to meet certain specified milestones or fulfill certain obligations, including to devote a certain amount of resources, in the development of our products. Failure to satisfy such obligations could result in the termination of our rights under such agreements.
If we choose to license our technology to third parties, this could result in disputes or otherwise limit our future operations.
We may also in the future, as one of our strategies, deploy our technology into the market and license patents and other intellectual proprietary rights to third parties. Disputes with our licensees may arise, including regarding the scope and content of these licenses. Additionally, a licensee may use our intellectual property without our permission, dispute our ownership of certain intellectual property rights or argue that our intellectual property does not cover our product. Regardless of whether we pursue legal action to enforce any such dispute, a dispute with a licensee or customer over intellectual property rights may damage our relationship with that licensee or customer and may also harm our reputation in the industry. Our ability to expand into additional fields with our technologies also may be restricted by licenses or other rights we may grant to third parties in the future, including if the licenses are exclusive, the licensee is assigned ownership of intellectual property that we develop or rights of first negotiation or refusal are granted.
We may in the future be subject to intellectual property rights claims, which are extremely costly to defend, could require us to pay significant damages and could limit our ability to use certain technologies.
Our success and ability to compete also depends in part on our ability to operate without infringing, misappropriating or otherwise violating the intellectual property or other proprietary rights of third parties. Companies in the technology industries, including some of our current and potential competitors, own large numbers of patents, copyrights, trademarks, and trade secrets and frequently pursue litigation based on allegations of infringement, misappropriation, or other violations of intellectual property rights. In addition, many of these companies have the capability to dedicate substantial resources to enforce their intellectual property rights and to defend claims that may be brought against them. Such litigation also may involve non-practicing patent assertion entities or companies who use their patents to extract license fees by threatening costly litigation or that have minimal operations or relevant product revenue and against whom our patents may provide little or no deterrence or protection. While we have not received any notices to date, we may receive notices in the future that claim we have infringed, misappropriated, misused, or otherwise violated other parties’ intellectual property rights, and, to the extent we become exposed to greater visibility, we face a higher risk of being the subject of intellectual property infringement, misappropriation or other violation claims, which is not uncommon with respect to software technologies in particular. There may be third-party intellectual property rights, including issued patents or pending patent applications, that cover significant aspects of our technologies, or business methods. There may also be third-party intellectual property rights, including trademark registrations and pending applications, that cover the goods and services that we offer in certain regions. We may also be exposed to increased risk of being the subject of intellectual property infringement, misappropriation, or other violation claims as a result of acquisitions and our incorporation of open source and other third-party software into, or new branding for, our software, as, among other things, we have a lower level of visibility into the development process with respect to such technology or the care taken to safeguard against infringement, misappropriation, or other violation risks. In addition, former employers of our current, former, or future employees may assert claims that such employees have improperly disclosed to us confidential or proprietary information of these former employers. Any intellectual property claims, with or without merit, are difficult to predict, could be very time-consuming and expensive to settle or litigate, could divert our management’s attention and other resources, and may not be covered by the insurance that we carry. These claims could subject us to significant liability for damages, potentially including treble damages if we are found to have willfully infringed a third party’s intellectual property rights. These claims could also result in our having to stop using technology, branding or marks found to be in violation of a third party’s rights and any necessary rebranding could result in the loss of goodwill. We could be required to seek a license for the intellectual property, which may not be available on commercially reasonable terms or at all. Even if a license were available, we could be required to pay significant royalties, which would increase our expenses. As a result, we could be required to develop alternative non-infringing technology, branding or marks, which could require significant effort and expense. If we cannot license rights or develop technology for any infringing aspect of our business, we would be forced to limit or stop sales of one or more of
87
our software or features, we could lose existing customers, and we may be unable to compete effectively. Any of these results would harm our business, financial condition, and results of operations.
Further, certain of our agreements with customers and other third parties may include indemnification provisions under which we agree to indemnify them for losses suffered or incurred as a result of third-party claims of intellectual property infringement, misappropriation, or other violations of intellectual property rights, damages caused by us to property or persons, or other liabilities relating to or arising from our software, services, or other contractual obligations. Large indemnity payments could harm our business, financial condition, and results of operations. Any dispute with a customer with respect to such obligations could have adverse effects on our relationship with that customer and other existing customers and new customers and harm our business and results of operations.
Risks Related to Our Management
We are highly dependent on key members of our executive management team. Our inability to retain these individuals could impede our business plan and growth strategies, which could have a negative impact on our business and the value of your investment.
Our ability to implement our business plan depends on the continued services of key members of our senior management. In particular, and to a critical extent, we are dependent on the continued efforts and services of the members of management named in the “Management” section. If we lose the services of such key members of our management team, we would likely be forced to expend significant time and money in the pursuit of replacement individuals, which may result in a delay in the implementation of our business plan and plan of operations. We may not be able to find satisfactory replacements on terms that would not be unduly expensive or burdensome to us. We do not currently carry a key-man life insurance policy that would assist us in recouping our costs in the event of the death or disability of a member of our management team. The loss of members of our management team, or our inability to attract or retain other qualified individuals, could have a material adverse effect on our business, results of operations and financial condition.
Our management team has limited experience managing a public company.
Other than our Chief Executive Officer, most members of our management team have limited or no experience managing a publicly traded company, interacting with public company investors and complying with the increasingly complex laws pertaining to public companies in the United States. Our management team may not successfully or efficiently manage our transition to being a public company subject to significant regulatory oversight and reporting obligations under the U.S. federal securities laws and the continuous scrutiny of securities analysts and investors. Their limited experience in dealing with the increasingly complex laws pertaining to public companies could be a significant disadvantage in that it is likely that an increasing amount of their time may be devoted to these activities which will result in less time being devoted to the management and growth of the Company. Thus, the Company may not have adequate personnel with the appropriate level of knowledge, experience, and training in the accounting policies, practices or internal controls over financial reporting required of public companies in the United States. The development and implementation of the standards and controls necessary for the Company to achieve the level of accounting standards required of a public company in the United States may require costs greater than expected. It is possible that the Company will be required to expand its employee base and hire additional employees to support its operations as a public company which will increase its operating costs in future periods. These new obligations and constituents will require significant attention from our senior management and could divert their attention away from the day-to-day management of our business, which could adversely affect our business, financial condition, results of operations and prospects.
Certain of our directors and/or officers may have interests that compete with ours.
Certain of our directors and/or officers currently own, operate and manage other entities, which may have similar or different objectives than ours. Such activities could detract from the time these people have to allocate to our affairs.
Our lack of adequate directors and officers insurance may also make it difficult for us to retain and attract talented and skilled directors and officers.
In the future we may be subject to litigation, including potential class action and shareholder derivative actions. Risks associated with legal liability are difficult to assess and quantify, and their existence and magnitude can remain unknown for significant periods of time. While we do have directors and officers (“D&O”) insurance it may not be sufficient in the case of litigation.
Moreover, the cost of maintaining adequate D&O insurance coverage may increase significantly in the future, our operating results could be materially adversely affected. Likewise, if any of our current D&O insurance coverage should
88
become unavailable to us or become economically impractical, we may need to decrease our coverage limits or increase our self-insured retention or we may be unable to renew such insurance at all. If we incur liabilities that exceed our coverage or incur liabilities not covered by our insurance, we would have to self-fund any indemnification amounts owed to our directors and officers and employees in which case our results of operations and financial condition could be materially adversely affected. Additionally, a lack of D&O insurance may make it difficult for us to retain and attract talented and skilled directors and officers to serve our company, which could adversely affect our business.
Risks Related to Ownership of Common Stock and Other Securities
The price of shares of common stock may be volatile or may decline regardless of our operating performance. You may lose some or all of your investment.
The trading price of shares of our common stock is likely to be volatile. The stock market recently has experienced extreme volatility. This volatility often has been unrelated or disproportionate to the operating performance of particular companies. You may not be able to resell your shares at an attractive price due to a number of factors such as those listed in “Risks Related to Our Business, Financial Condition, and Need for Additional Capital” and the following:
•our operating and financial performance and prospects;
•quarterly or annual earnings or those of other companies in our industry compared to market expectations;
•conditions that impact demand for our products and/or services;
•future announcements concerning our business, our clients’ businesses or our competitors’ businesses;
•the public’s reaction to our press releases, other public announcements and filings with the SEC;
•the market’s reaction to our reduced disclosure and other requirements as a result of being an “emerging growth company” under the Jumpstart Our Business Startups Act of 2012 (“JOBS Act”);
•the size of our public float;
•coverage by or changes in financial estimates by securities analysts or failure to meet their expectations;
•market and industry perception of our success, or lack thereof, in pursuing our growth strategy;
•strategic actions by us or our competitors, such as acquisitions or restructurings;
•changes in laws or regulations which adversely affect our industry or us;
•privacy and data protection laws, privacy or data breaches, or the loss of data;
•changes in accounting standards, policies, guidance, interpretations or principles;
•changes in senior management or key personnel;
•issuances, exchanges or sales, or expected issuances, exchanges or sales of our capital stock;
•changes in our dividend policy;
•adverse resolution of new or pending litigation against us; and
•changes in general market, economic and political conditions in the United States and global economies or financial markets, including those resulting from natural disasters, terrorist attacks, acts of war and responses to such events.
These broad market and industry factors may materially reduce the market price of shares of common stock, regardless of our operating performance. In addition, price volatility may be greater if the public float and trading volume of common stock is low. A reduced trading volume may result now that our common stock is not trading on Nasdaq and is instead trading on the OTC Markets’ OTCQB Venture Market tier. As a result, you may suffer a loss on your investment.
In the past, following periods of market volatility, stockholders have instituted securities class action litigation. If we were involved in securities litigation, it could have a substantial cost and divert resources and the attention of executive management from our business regardless of the outcome of such litigation.
89
We do not intend to pay dividends on shares of common stock for the foreseeable future.
We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, we do not anticipate declaring or paying any cash dividends on shares of common stock in the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of our Board of Directors (the “Board”) and will depend on, among other things, our business prospects, results of operations, financial condition, cash requirements and availability, certain restrictions related to our indebtedness, industry trends and other factors that the Board may deem relevant. Any such decision will also be subject to compliance with contractual restrictions and covenants in the agreements governing our current and future indebtedness. In addition, we may incur additional indebtedness, the terms of which may further restrict or prevent us from paying dividends on common stock. As a result, you may have to sell some or all of your shares of common stock after price appreciation in order to generate cash flow from your investment, which you may not be able to do. Our inability or decision not to pay dividends, particularly when others in our industry have elected to do so, could also adversely affect the market price of shares of common stock.
If securities analysts do not publish research or reports about us, or if they issue unfavorable commentary about us or our industry or downgrade our common stock, the price of shares of common stock could decline.
The trading market for shares of common stock will depend in part on the research and reports that third-party securities analysts publish about us and the industries in which we operate. We may be unable or slow to attract research coverage and if one or more analysts cease coverage of us, the price and trading volume of our securities would likely be negatively impacted. If any of the analysts that may cover us change their recommendation regarding our securities adversely, or provide more favorable relative recommendations about our competitors, the price of our securities would likely decline. If any analyst that may cover us ceases covering us or fails to regularly publish reports on us, we could lose visibility in the financial markets, which could cause the price or trading volume of our securities to decline. Moreover, if one or more of the analysts who cover us downgrades the common stock, or if our reporting results do not meet their expectations, the market price of shares of common stock could decline.
Our issuance of additional shares of common stock or securities convertible or exchangeable into common stock could make it difficult for another company to acquire us, may dilute your ownership of us and could adversely affect our stock price.
From time to time in the future, we may also issue additional shares of common stock or securities convertible into common stock pursuant to a variety of transactions, including acquisitions. The issuance by us of additional shares of common stock or securities convertible into common stock would dilute your ownership of us and the sale of a significant amount of such shares in the public market could adversely affect prevailing market prices of shares of common stock. In the future, we expect to obtain financing or to further increase our capital resources by issuing additional shares of our capital stock or offering debt or other equity securities, including senior or subordinated notes, debt securities convertible into equity, or shares of preferred stock. Issuing additional shares of our capital stock, other equity securities, or securities convertible into equity may dilute the economic and voting rights of our existing stockholders, reduce the market price of shares of common stock, or both. Debt securities convertible into equity could be subject to adjustments in the conversion ratio pursuant to which certain events may increase the number of equity securities issuable upon conversion. Preferred stock, if issued, could have a preference with respect to liquidating distributions or a preference with respect to dividend payments that could limit our ability to pay dividends to the holders of our common stock. Our decision to issue securities in any future offering will depend on market conditions and other factors beyond our control, which may adversely affect the amount, timing or nature of our future offerings. As a result, holders of common stock bear the risk that our future offerings may reduce the market price of shares of common stock and dilute their percentage ownership.
Future sales, or the perception of future sales, of common stock by us or our existing stockholders in the public market could cause the market price for our common stock to decline.
The sale of substantial amounts of shares of common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock. These sales, or the possibility that these sales may occur, also might make it more difficult for us to sell equity securities in the future at a time and at a price that we deem appropriate.
All shares issued as merger consideration in the Business Combination are freely tradable without registration under the Securities Act of 1933, as amended (the “Securities Act”) and without restriction by persons other than our “affiliates” (as defined under Rule 144), including our directors, executive officers and other affiliates, and certain other former QT Imaging stockholders.
90
The Company has registered in a resale registration statement on Form S-1 declared effective by the SEC on May 22, 2024, securities held by certain stockholders of the Company which have the right, subject to certain conditions, to require us to register the sale of their shares of common stock under the Securities Act, pursuant to the terms of a registration statement that we entered into with GigAcquisitions5 and certain other securityholders of GigCapital5 and QT Imaging (the “GigCapital5 Registration Rights Agreement”). The Company has also registered in a resale registration statement on From S-1 declared effective by the SEC on February 5, 2025, the securities sold in the Private Placement as the purchaser received the right, subject to certain conditions, to require us to register the sale of their shares of common stock under the Securities Act, pursuant to the terms of of a registration statement that we entered into (“PIPE Registration Rights Agreement”). The sale of a large number of shares by these stockholders could cause the prevailing market price of shares of common stock to decline. Significant sales of shares of common stock may have negative pressure on the public trading price of our common stock.
The shares already registered for resale currently represent over 50% of the total number of shares outstanding, based on the number of shares of common stock outstanding as of March 15, 2025. Even though the current trading price is significantly below the Company’s initial public offering price, based on the closing price of the common stock on March 28, 2025, certain of our stockholders may have an incentive to sell their shares because they will still profit on sales due to the lower prices at which they purchased their shares as compared to the public investors. For example, members of our founding stockholder, GigAcquisitions5, who received a distribution of shares from GigAcquisitions5 for which there is an effective resale registration statement, have a cost basis in up to 5,735,000 shares of common stock that were acquired at an effective purchase price of $0.0043592 per share, and, therefore, based on the closing price of the common stock on March 28, 2025, could earn an aggregate profit of approximately $2,985,875 from the resale of such shares.
To the extent that the selling securityholders under any of our registration statements on Form S-1 sell their shares or are perceived by the market as intending to sell them, the market price of shares of common stock could drop significantly. These factors could also make it more difficult for us to raise additional funds through future offerings of shares of common stock or other securities.
As of December 31, 2024, the Company had 23,889,364 warrants outstanding that were exercisable for cash at an exercise price of $2.30 per share, and an additional 4,383,558 warrants outstanding that would become exercisable for cash on May 22, 2025 with an exercise price of $0.672 per share. On February 26, 2025, the Company issued an additional 61,000,000 warrants to Lynrock Lake and 15,000,000 warrants to YA II PN, Ltd. which are exercisable on a "cashless basis" with an exercise price of $0.40 per share as part of the $10.1 million term loan received from Lynrock Lake and cancellation of debt with YA II PN, Ltd. The exercise of any of these warrants would result in the issuance of shares which could then be sold.
In addition, the shares of common stock that will be issued upon exercise of stock options already granted pursuant to the terms of, or those shares of common stock reserved for future issuance under the 2024 Equity Incentive Plan will become eligible for sale in the public market once those shares are issued, subject to provisions relating to various vesting agreements and, in some cases, limitations on volume and manner of sale applicable to affiliates under Rule 144, as applicable. We have filed a registration statement on Form S-8 under the Securities Act to register shares of common stock or securities convertible into or exchangeable for shares of common stock issued pursuant to our equity incentive plans, and may in the future file additional registration statements on Form S-8. Any such Form S-8 registration statements will automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
As a public reporting company, we are subject to rules and regulations established from time to time by the SEC regarding our internal control over financial reporting.
The Company is a public reporting company subject to the rules and regulations established from time to time by the SEC. These rules and regulations will require, among other things that the Company establish and periodically evaluate procedures with respect to its internal control over financial reporting. Reporting obligations as a public company are likely to place a considerable strain on the Company’s financial and management systems, processes and controls, as well as on its personnel.
In addition, as a public company, the Company will be required to document and test its internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act so that its management can certify as to the effectiveness of the internal control over financial reporting. If the Company’s not able to implement the requirements of Section 404, including any additional requirements once the Company’s no longer an emerging growth company, in a timely manner or with adequate compliance, it may not be able to assess whether its internal control over financial reporting are effective,
91
which may subject the Company to adverse regulatory consequences and could harm investor confidence and the market price of our common stock.
Additionally, once we are no longer an emerging growth company, we will be required to comply with the independent registered public accounting firm attestation requirement on our internal control over financial reporting. We will be an “emerging growth company” until the earlier of (1) the last day of the fiscal year (a) following September 28, 2026, the fifth anniversary of our initial public offering (“IPO”), (b) in which we have total annual gross revenue of at least $1.235 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our prior second fiscal quarter, and (2) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. Until we cease being an emerging growth company stockholders will not have the benefit of an independent assessment of the effectiveness of our internal control environment.
As an “emerging growth company,” we cannot be certain if the reduced disclosure requirements applicable to “emerging growth companies” will make our common stock less attractive to investors.
As an “emerging growth company,” we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to obtain an assessment of the effectiveness of our internal controls over financial reporting from our independent registered public accounting firm pursuant to Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirements of holding a non-binding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
In addition, Section 102(b)(1) of the JOBS Act exempts emerging growth companies from being required to comply with new or revised financial accounting standards until private companies (that is, those that have not had a Securities Act registration statement declared effective or do not have a class of securities registered under the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) are required to comply with the new or revised financial accounting standards. The JOBS Act provides that a company can elect to opt out of the extended transition period and comply with the requirements that apply to non-emerging growth companies but any such an election to opt out is irrevocable. The Company has elected not to opt out of such extended transition period which means that when a standard is issued or revised and it has different application dates for public or private companies, the Company, as an emerging growth company, can adopt the new or revised standard at the time private companies adopt the new or revised standard. This may make comparison of the Company’s financial statements with another public company which is neither an emerging growth company nor an emerging growth company which has opted out of using the extended transition period difficult or impossible because of the potential differences in accountant standards used.
We cannot predict if investors will find our common stock less attractive because we will rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active market for our common stock, our share price may be more volatile and the price at which our securities trade could be less than if we did not use these exemptions.
Anti-takeover provisions in our governing documents and under Delaware law could make an acquisition of us more difficult, limit attempts by our stockholders to replace or remove our current management and limit the market price of our common stock.
The Second Amended and Restated Certificate of Incorporation of the Company (the “Charter”), the Company’s bylaws (the “Bylaws”) and Delaware law contain provisions that could have the effect of rendering more difficult, delaying, or preventing an acquisition deemed undesirable by the Board. Among other things, the Charter and/or the Bylaws include the following provisions:
•a staggered board, which means that the Board will be classified into three classes of directors with staggered three-year terms and directors will only be able to be removed from office for cause;
•limitations on convening special stockholder meetings, which could make it difficult for our stockholders to adopt desired governance changes;
•prohibition on stockholder action by written consent, which means that our stockholders will only be able to take action at a meeting of stockholders and will not be able to take action by written consent for any matter;
•a forum selection clause, which means certain litigation against us can only be brought in Delaware;
92
•the authorization of undesignated preferred stock, the terms of which may be established and shares of which may be issued without further action by our stockholders; and
•advance notice procedures, which apply for stockholders to nominate candidates for election as directors or to bring matters before an annual meeting of stockholders.
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. As a Delaware corporation, we are also subject to provisions of Delaware law, including Section 203 of the Delaware General Corporation Law (“DGCL”), which prevents interested stockholders, such as certain stockholders holding more than 15% of our outstanding common stock, from engaging in certain business combinations unless (i) prior to the time such stockholder became an interested stockholder, the board of directors approved the transaction that resulted in such stockholder becoming an interested stockholder, (ii) upon consummation of the transaction that resulted in such stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the common stock, or (iii) following board approval, such business combination receives the approval of the holders of at least two-thirds of our outstanding common stock not held by such interested stockholder.
Any provision of the Charter, the Bylaws or Delaware law that has the effect of delaying, preventing or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock and could also affect the price that some investors are willing to pay for our common stock.
The Charter provides that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers, or employees.
The Charter provides that, unless we consent in writing to the selection of an alternative forum, the (i) Delaware Court of Chancery (the “Court of Chancery”) of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for: (A) any derivative action, suit or proceeding brought on our behalf; (B) any action, suit or proceeding asserting a claim of breach of fiduciary duty owed by any of our directors, officers, or stockholders to us or to our stockholders; (C) any action, suit or proceeding asserting a claim arising pursuant to the DGCL, the Charter or the Bylaws; or (D) any action, suit or proceeding asserting a claim governed by the internal affairs doctrine; and (ii) subject to the foregoing, the federal district courts of the United States of America shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. Notwithstanding the foregoing, such forum selection provisions shall not apply to suits brought to enforce any liability or duty created by the Exchange Act or any other claim for which the federal courts of the United States have exclusive jurisdiction. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers, or other employees, which may discourage such lawsuits against us and our directors, officers, and other employees. Alternatively, if a court were to find the choice of forum provision contained in the Charter to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, results of operations, and financial condition.
Additionally, Section 22 of the Securities Act creates concurrent jurisdiction for federal and state courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations thereunder. As noted above, the Charter provides that the federal district courts of the United States of America shall have jurisdiction over any action arising under the Securities Act. Accordingly, there is uncertainty as to whether a court would enforce such provision. Our stockholders will not be deemed to have waived our compliance with the federal securities laws and the rules and regulations thereunder.
If we do not file and maintain a current and effective prospectus relating to the common stock issuable upon exercise of the public and private warrants, holders will only be able to exercise such warrants on a "cashless basis" and we have a large number of other warrants which are exercisable on a “cashless basis.”
If we do not file and maintain a current and effective prospectus relating to the common stock issuable upon exercise of the public and private warrants at the time that holders wish to exercise such warrants, they will only be able to exercise them on a “cashless basis” provided that an exemption from registration is available. As a result, the number of shares of common stock that holders will receive upon exercise of the public and private warrants will be fewer than it would have been had such holder exercised its warrant for cash. Further, if an exemption from registration is not available, holders would not be able to exercise on a cashless basis and would only be able to exercise their warrants for cash if a current and effective prospectus relating to the common stock issuable upon exercise of the warrants is available. Under the terms of the Warrant Agreement, dated as of September 23, 2021, between GigCapital5 and the Transfer Agent (the “Warrant Agreement”), we have agreed to use our best efforts to meet these conditions and to file and maintain a current and
93
effective prospectus relating to the common stock issuable upon exercise of the warrants until the expiration of the warrants. However, we cannot assure you that we will be able to do so. If we are unable to do so, the potential “upside” of the holder’s investment in us may be reduced or the warrants may expire worthless.
In addition, on February 26, 2025, we issued an additional 61,000,000 warrants to Lynrock Lake and 15,000,000 warrants to YA II PN, Ltd. which are exercisable on a "cashless basis" with an exercise price of $0.40 per share as part of the $10.1 million term loan received from Lynrock Lake and cancellation of debt with YA II PN, Ltd..
There is no guarantee that the public and private warrants will ever be in the money, and they may expire worthless and the terms of warrants may be amended.
The exercise price for the public and private warrants is $2.30 per share of common stock. There is no guarantee that the public and private warrants will ever be in the money prior to their expiration, and as such, the warrants may expire worthless.
In addition, the Company’s public and private warrants were issued in registered form under the Warrant Agreement between Continental Stock Transfer & Trust Company, as warrant agent, and the Company. The Warrant Agreement provides that the terms of the warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any other change. Accordingly, the Company may amend the terms of the warrants in a manner adverse to a holder if holders of at least 50% of the then outstanding public warrants approve of such amendment. Although the Company’s ability to amend the terms of the warrants with the consent of at least 50% of the then outstanding public warrants is unlimited, examples of such amendments could be amendments to, among other things, increase the exercise price of the warrants, shorten the exercise period or decrease the number of shares and their respective affiliates and associates have of common stock purchasable upon exercise of a warrant.
Our warrants will become exercisable for the common stock, which would increase the number of shares eligible for future resale in the public market and result in dilution to our stockholders.
Our private warrants and our public warrants issued as part of GigCapital5’s IPO are exercisable for one share of common stock at $2.30 per share. Our PIPE Warrants are exercisable for one share of stock at $0.672 per share. Warrants that we have issued in February 2025 to Lynrock Lake (as defined below) and YA II PN, Ltd are exercisable for one share at $0.40 per share. The additional shares of common stock issued upon exercise of our warrants will result in dilution to the then existing holders of the common stock and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market could adversely affect the market price of the common stock.
We have no obligation to net cash settle the warrants other than the warrants issued to Lynrock Lake and YA II PN, Ltd.
In no event will we have any obligation to net cash settle the warrants other than the warrants issued to Lynrock Lake and YA II PN, Ltd. Furthermore, there are no contractual penalties for failure to deliver securities to the holders of the public and private warrants upon exercise of those warrants. Accordingly, those warrants may expire worthless.
Our public warrants, private warrants, and the warrants issued to Lynrock Lake and YA II PN, Ltd are, and our PIPE Warrants will become, exercisable for common stock, which would increase the number of shares eligible for future resale in the public market and result in dilution to our stockholders.
Outstanding public warrants to purchase an aggregate of 23,000,000 shares of common stock are exercisable in accordance with the terms of the Warrant Agreement governing those securities, as well as our private warrants to purchase an aggregate of up to 889,364 shares of common stock, at an exercise price of $2.30 per share. The warrants issued to Lynrock Lake and YA II PN, Ltd to purchase an aggregate of 76,000,000 shares of common stock at an exercise price of $0.40 per share are also exercisable. In addition, the PIPE Warrants will become exercisable as of May 22, 2025 at an exercise price of $0.672 per share. To the extent such warrants are exercised, additional shares of common stock will be issued, which will result in dilution to the holders of common stock and increase the number of shares eligible for resale in the public market. Sales of substantial numbers of such shares in the public market or the fact that such warrants may be exercised could adversely affect the market price of common stock. However, there is no guarantee that the warrants will ever be in-the-money prior to their expiration. As such, the warrants may expire worthless.
94
Certain of the Company’s warrants are accounted for as a warrant liability and were recorded at fair value upon issuance with changes in fair value each period reported in earnings, which may have an adverse effect on the market price of the common stock.
As of December 31, 2024, 889,364 private warrants were outstanding. These warrants became exercisable 30 days after completion of the Business Combination and are exercisable now that we have an effective registration statement under the Securities Act covering the shares of common stock of the Company issuable upon exercise for so long as a current prospectus relating to them is available and such shares are registered, qualified or exempt from registration under the securities, or blue sky, laws of the state of residence of the holder (or the Company permits holders to exercise their warrants on a cashless basis under certain circumstances). Furthermore, the Company may redeem outstanding warrants in certain circumstances; provided, however, that these warrants will not be redeemable by the Company so long as they are held by the initial purchasers or any of its permitted transferees, to which the initial purchaser transferred the private warrants in June 2024. Under GAAP, the Company is required to evaluate contingent exercise provisions of these warrants and then their settlement provisions to determine whether they should be accounted for as a warrant liability or as equity. Any settlement amount not equal to the difference between the fair value of a fixed number of the Company’s equity shares and a fixed monetary amount precludes these warrants from being considered indexed to its own stock, and therefore, from being accounted for as equity. As a result of the provision that these warrants, when held by someone other than the initial purchasers or their permitted transferees, will be redeemable by the Company, the requirements for accounting for these warrants as equity are not satisfied. Therefore, the Company is required to account for these warrants as a warrant liability and record (a) that liability at fair value, and (b) any subsequent changes in fair value as of the end of each period for which earnings are reported. The impact of changes in fair value on earnings may have an adverse effect on the market price of our common stock.
Our common stock will no longer be listed on The Nasdaq Global Market
Our common stock is currently listed on The Nasdaq Global Market. Continued listing of the common stock on Nasdaq requires satisfying certain criteria. Violating the Nasdaq’s listing requirements or failing to meet its listing standards means that our common stock will be delisted.
On May 10, 2024, we received a written notice from the Listing Qualifications Department (the “Staff”) of Nasdaq notifying us that, for the 30 consecutive business days prior to May 6, 2024, our Market Value of Listed Securities (“MVLS”) was below the minimum of $50 million required for continued listing on The Nasdaq Global Market pursuant to Nasdaq Listing Rule 5450(b)(2)(A) (the “MVLS Requirement”).
Furthermore, on June 17, 2024, the Staff notified us that the minimum bid price of our common stock had been below $1.00 per share for 30 consecutive business days, and, as a result, did not comply with Listing Rule 5450(a)(1) of the Nasdaq Listed Company Manual (the “Price Rule”). In accordance with Listing Rule 5810(c)(3)(A), we were provided with 180 calendar days, or until December 16, 2024, to regain compliance with the Price Rule.
In addition, on September 10, 2024, the Staff notified us that, for the prior 31 consecutive business days, our Market Value of Publicly Held Securities (“MVPHS”) was below the minimum of $15 million required for continued listing on The Nasdaq Global Market pursuant to Nasdaq Listing Rule 5450(b)(2)(c) (the “MVPHS Requirement”).
Subsequently, the Staff notified us on November 6, 2024 that it had determined to commence proceedings to delist the common stock from Nasdaq due to its determination that our common stock is no longer suitable for listing because our market value of our listed securities fell below the minimum $50 million required for continued listing as set forth in MVLS Requirement, and we were unable to regain compliance with the MVLS Requirement by November 4, 2024. We proceeded to initiate an appeal (the “Appeal”) of the Staff’s determination to commence delisting of the common stock from Nasdaq, requesting that the matter be submitted to a Hearings Panel (the “Panel”) per the procedures set forth in the Nasdaq Listing Rule 5800 Series, staying the suspension of our securities and the filing of a Form 25‑NSE by the Staff pending the Panel’s decision.
While the Appeal was pending, on December 17, 2024, the Staff formally notified us that we were unable to regain compliance with the Price Rule during the provided 180-day compliance window, which the Staff stated it considers an additional basis for delisting our common stock from Nasdaq and which was to be considered in the Panel’s rendering of a decision on the Appeal.
On January 7, 2025, the Panel held a hearing on the Appeal of the Staff’s November 6, 2024 and December 17, 2024 decisions to commence proceedings to delist our common stock. On January 24, 2025, we received further notice that the Panel had denied the Appeal and that our common stock will be delisted from trading on Nasdaq based on the failure to comply with the MVLS Requirement and the Price Rule. Accordingly, our common stock was suspended from trading on
95
Nasdaq effective with the open of trading on January 28, 2025. Our common stock will be delisted 10 calendar days from the date that Nasdaq files the Form 25, Notification of Removal from Listing and/or Registration, with the SEC. Commencing on January 28, 2025, our common stock commenced trading on the over-the-counter (OTC) Pink Sheets under the ticker “QTIH”, and it was upgraded to trading on the OTC Markets’ OTCQB Venture Market tier effective March 11, 2025. If in the future, it is able to qualify to list on Nasdaq under the Nasdaq’s initial listing standards, we intend to apply for listing on Nasdaq.
We do not expect the Panel’s determination to have any impact on our day-to-day operations. However, we believe that delisting of our common stock from Nasdaq may adversely affect our ability to raise additional financing through the public or private sale of equity securities, may significantly affect the ability of investors to trade our securities and may negatively affect the value and liquidity of our common stock. Delisting could have other negative results, including the potential loss of employee confidence, the loss of institutional investors and/or interest in significant business development opportunities.
By trading on the OTC Markets OTCQB Venture Market tier, we could face significant adverse consequences including, among others:
•a limited availability of market quotations for our securities;
•a limited amount of news and little or no analyst coverage of us;
•we would no longer qualify for exemptions from state securities registration requirements, which may require us to comply with applicable state securities laws; and
•a decreased ability to issue additional securities (including pursuant to short-form registration statements on Form S-3) or obtain additional financing in the future.
In addition, an increase in the per share trading value of our common stock would be beneficial because it would:
•improve the perception of our common stock as an investment security;
•reset our stock price to more normalized trading levels in the face of potentially extended market dislocations;
•assist with future potential capital raises;
•appeal to a broader range of investors to generate greater investor interest in us; and
•reduce stockholder transaction costs because investors would pay lower commissions to trade a fixed dollar amount of our common stock if our stock price were higher than they would if our stock price were lower.
Trading on the OTCQB Venture Market tier is volatile and sporadic, which could depress the market price of our common stock and make it difficult for our stockholders to resell their shares of our common stock.
Our common stock is now quoted on the OTC Markets OTCQB Venture Market tier. Trading in securities quoted on the OTC Markets is often thin and characterized by wide fluctuations in trading prices, due to many factors, some of which may have little to do with our operations or business prospects. This volatility could depress the market price of our common stock for reasons unrelated to operating performance. Moreover, the OTC Markets is not a stock exchange, and trading of securities on the OTC Markets is often more sporadic than the trading of securities listed on a quotation system like The Nasdaq Global Market, where our common stock has been listed since the Business Combination. These factors may result in investors having difficulty reselling any shares of our common stock.
Our common stock is quoted on the OTCQB Venture Market tier, which may have an unfavorable impact on our stock price and liquidity.
Our common stock is quoted on the OTCQB Venture Market tier. The OTCQB Venture Market tier is a significantly more limited market than Nasdaq. The quotation of our shares on the OTCQB Venture Market tier may result in a less liquid market available for existing and potential stockholders to trade shares of our common stock, could depress the trading price of our common stock and could have a long-term adverse impact on our ability to raise capital in the future. We plan to relist our common stock as soon as practicable on a national stock exchange. However, we cannot assure you
96
that we will be able to meet the initial listing standards of any stock exchange, or that we will be able to maintain any such listing.
Other General Risks Applicable to the Company
Under applicable employment laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees.
Our employment agreements generally include covenants not to compete. These agreements prohibit our employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work at all or for a sufficient duration of time to prevent members of our management team from competing with us.
We may not be able to attract and retain the highly skilled employees we need to support our planned growth.
To continue to execute our business and our growth plan, we must attract and retain highly qualified personnel. Competition for these personnel is intense. We may not be successful in attracting and retaining qualified personnel. If we fail to attract new personnel or fail to retain and motivate our current personnel, our business, financial condition, results of operations and future growth prospects could be severely harmed.
Industry data, projections and estimates relied upon by us are inherently uncertain, subject to interpretation and may not have been independently verified.
Information concerning our industry and the markets in which we operate and intend to operate, including industry projections and estimates, is obtained from publicly available information released by independent industry and research organizations and other third-party sources. We have not independently verified any such third-party information. In addition, projections, assumptions and estimates of the future performance of the industry in which we operate are subject to uncertainty and risk due to a variety of factors. As a result, inaccuracies in third-party information, or in the projections, may adversely impact the assumptions that are relied upon for our internal business planning and in the analysis of investors.
We will incur increased costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could adversely affect the Company’s business, results of operations, and financial condition.
As a public company, we will incur significant legal, accounting and other expenses that the company did not incur as a private company, including costs associated with public company reporting requirements. We will also incur costs associated with corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), as well as rules implemented by the SEC and the Nasdaq. These rules and regulations are expected to increase the company’s legal and financial compliance costs and to make some activities more time consuming and costly, which may adversely affect investor confidence and could cause our business or stock price to suffer.
Certain estimates of market opportunity included in this annual report may prove to be inaccurate.
This annual report includes our internal estimates of the addressable market for our products. Market opportunity estimates, whether obtained from third-party sources or developed internally, are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. The estimates in this annual report relating to the size of our target market, market demand and adoption, capacity to address this demand, and pricing may prove to be inaccurate. The addressable market we estimate may not materialize for many years, if ever, and even if the markets in which we compete meet the size estimates in this annual report, our business could fail to successfully address or compete in such markets.
We may be required to take write-downs or write-offs, restructuring and impairment or other charges that could have a significant negative effect on our financial condition, results of operations and stock price, which could cause you to lose some or all of your investment.
We may be forced to later write-down or write-off assets, restructure our operations, or incur impairment or other charges that could result in losses if material issues are discovered or factors that are outside of our control subsequently arise that require us to do so. Unexpected risks may arise and previously known risks may materialize in a manner not consistent with our existing risk analysis. Even though these charges may be non-cash items and may not have an immediate impact on our liquidity, the fact that we report charges of this nature could contribute to negative market
97
perceptions about us or our securities. In addition, charges of this nature may cause us to be unable to obtain future financing on favorable terms or at all.
Exchange rate fluctuations between the U.S. dollar and other currencies and inflation may negatively affect our results of operations, and we may not be able to hedge our currency exchange risks successfully.
The U.S. dollar is our functional and reporting currency. Payments we receive from international distribution partners and others that purchase our products and services may be subject to currency fluctuations if the remitting party does not initiate payment in U.S. dollars. Given our general lack of currency hedging arrangements to protect us from fluctuations in the exchange rates of foreign currencies in relation to the U.S. dollar (and/or from inflation of such foreign currencies), we may be exposed to material adverse effects from such movements. Our exchange rate exposure may change over time as our business evolves and could result in increased costs or reduced revenue and could affect our actual cash flow. Changes in the relative values of currencies occur regularly and, in some instances, may have a significant impact on our operating results. The rate of inflation in countries in which we sell and service our products, or in currency exchange rates, may materially change and we might not be able to effectively mitigate these risks.
We will incur significant increased expenses and administrative burdens as a public company, which could have an adverse effect on its business, financial condition and results of operations.
We face increased legal, accounting, administrative and other costs and expenses as a public company than the Company did as a private company. The Sarbanes-Oxley Act, including the requirements of Section 404, as well as rules and regulations subsequently implemented by the SEC, the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 and the rules and regulations promulgated and to be promulgated thereunder, Public Company Accounting Oversight Board (United States) (the “PCAOB”) and the securities exchanges, impose additional reporting and other obligations on public companies. Compliance with public company requirements will increase costs and make certain activities more time-consuming. A number of those requirements require the Company to carry out activities QT Imaging has not done previously. For example, the Company created new board committees and adopted new internal controls and disclosure controls and procedures. In addition, expenses associated with SEC reporting requirements will be incurred. Furthermore, if any issues in complying with those requirements are identified (for example, if the auditors identify any additional material weaknesses or significant deficiencies in the internal control over financial reporting), the Company could incur additional costs rectifying those issues, and the existence of those issues could adversely affect the Company reputation or investor perceptions of it. It may also be more expensive to obtain director and officer liability insurance. Risks associated with the Company’ status as a public company may make it more difficult to attract and retain qualified persons to serve on our Board or as executive officers. The additional reporting and other obligations imposed by these rules and regulations will increase legal and financial compliance costs and the costs of related legal, accounting and administrative activities. These increased costs will require the Company to divert a significant amount of money that could otherwise be used to expand the business and achieve strategic objectives. Advocacy efforts by stockholders and third parties may also prompt additional changes in governance and reporting requirements, which could further increase costs.
Effective internal controls are necessary for us to provide reliable financial reports and prevent fraud.
If we identify any additional material weaknesses in the future, any such identified material weakness could limit our ability to prevent or detect a misstatement of our accounts or disclosures that could result in a material misstatement of our annual or interim consolidated financial statements. In such case, we may be unable to maintain compliance with securities law requirements regarding timely filing of periodic reports in addition to applicable stock exchange listing requirements, investors may lose confidence in our financial reporting and our stock price may decline as a result. We cannot assure you that the measures we have taken to date, or any measures we may take in the future, will be sufficient to avoid potential future material weaknesses.
Unanticipated changes in effective tax rates or adverse outcomes resulting from examination of our income or other tax returns could adversely affect our financial condition and results of operations.
We will be subject to income taxes in the United States and other jurisdictions, and our tax liabilities will be subject to the allocation of expenses in differing jurisdictions. Our future effective tax rates could be subject to volatility or adversely affected by a number of factors, including:
•changes in the valuation of our deferred tax assets and liabilities;
•expected timing and amount of the release of any tax valuation allowances;
•tax effects of stock-based compensation;
98
•costs related to intercompany restructurings;
•changes in tax laws, regulations or interpretations thereof; or
•lower than anticipated future earnings in jurisdictions where we have lower statutory tax rates and higher than anticipated future earnings in jurisdictions where we have higher statutory tax rates.
In addition, we may be subject to audits of our income, sales and other transaction taxes by taxing authorities. Outcomes from these audits could have an adverse effect on our financial condition and results of operations.
Our only significant asset is its ownership interest in QT Imaging, Inc. and such ownership may not be sufficient to pay dividends or make distributions or loans to enable the Company to pay any dividends on the common stock or satisfy its other financial obligations.
We have no direct operations and no significant assets other than its ownership of QT Imaging. We depend on QT Imaging for distributions, loans and other payments to generate the funds necessary to meet our financial obligations, including our expenses as a publicly traded company and to pay any dividends with respect to our common stock. The financial condition and operating requirements of QT Imaging may limit our ability to obtain cash from QT Imaging. The earnings from, or other available assets of, QT Imaging may not be sufficient to pay dividends or make distributions or loans to enable us to pay any dividends on the common stock or satisfy its other financial obligations.
Our ability to make distributions, loans and other payments for the purposes described above and for any other purpose may be limited by credit agreements to which the Company is party from time to time, including the existing loan agreement with Lynrock Lake, and will be subject to the negative covenants set forth therein. Any loans or other extensions of credit to QT Imaging from the Company will be permitted only to the extent there is an applicable exception to the investment covenants under this loan agreement. Similarly, any dividends, distributions or similar payments to QT Imaging from the Company will be permitted only to the extent there is an applicable exception to the dividends and distributions covenants under this loan agreement.
Item 1B: Unresolved Staff Comments
None
Item 1C: Cybersecurity
Cyber-based attacks can include computer viruses, denial-of-service attacks, phishing attacks, ransomware attacks and other introduction of malware to computers and networks; unauthorized access through the use of compromised credentials; exploitation of design flaws, bugs or security vulnerabilities; intentional or unintentional acts by employees or other insiders with access privileges; and intentional acts of vandalism by third parties and sabotage.
We have developed and implemented a risk-based program focused on assessing and identifying cybersecurity risks, and managing these risks by taking cybersecurity measures to protect the confidentiality, integrity, and availability of our critical systems and information.
99
We continue to assess the risks and changes in the cyber environment and invest in enhancements to our cybersecurity capabilities.
Item 2: Properties
We currently maintain our corporate offices at 3 Hamilton Landing, Suite 160, Novato, CA.
Item 3: Legal Proceedings
We are not currently subject to any material legal proceedings, nor, to our knowledge, is any material legal proceeding threatened against us or any of our officers or directors in their corporate capacity.
Item 4: Mine Safety Disclosures
Not applicable
100
Part II
Item 5: Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities
Our common stock is currently listed on The Nasdaq Stock Market LLC. However, the Company has received written notice from Nasdaq that it has commenced proceedings to delist the Company’s common (ticker symbol: QTI) from Nasdaq, and suspended trading in the Company’s common stock pending the completion of such proceedings. As a result, effective January 28, 2025, the Company’s common stock commenced trading in the over‑the-counter market under the symbol “QTIH”, and the trading of the common stock was upgraded to the OTCQB Venture Market on March 11, 2025. Any over-the-counter market quotations of our common stock reflect inter-dealer prices, without retail mark-up, mark-down, or commission and may not necessarily represent actual transactions.
As of March 28, 2025, there were approximately 384 stockholders of record of our common stock.
Dividend Policy
We have never declared or paid any cash dividend on our common stock. We currently do not expect to pay any dividends on our common stock in the foreseeable future.
Recent Unregistered Sales of Equity Securities
Please refer to the Quarterly Report on Form 10-Q and registration statement on Form S-1 that we have filed with the SEC for information regarding our Private Placement that closed on November 22, 2024.
Recent Purchases of Equity Securities
We made no repurchases of our equity securities during the fourth quarter of the fiscal year ended December 31, 2024.
Equity Compensation Plans
See Item 12, Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters, of Part III of this Annual Report for information relating to securities authorized for issuance under our equity compensation plans.
Item 6: Reserved
Item 7: Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis provides information that our management believes is relevant to an assessment and understanding of our consolidated results of operations and financial condition. The discussion should be read in conjunction with our audited consolidated financial statements, including the notes thereto, attached hereto.
This discussion contains forward-looking statements based upon our management’s current beliefs and expectations that involve risks, uncertainties, assumptions and other factors that could cause actual results to differ materially from those made or implied in the forward-looking statements. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those discussed below and those set forth under “Risk Factors” and elsewhere in this annual report. Accordingly, you are cautioned not to place undue reliance on these forward-looking statements, which reflect our management’s analysis only as of the date hereof.
Unless the context otherwise requires, references in this “Management’s Discussion and Analysis of Condition and Results of Operations” to “we,” “our,” “us,” “QT Imaging,” and the “Company” refer to the business and operations of QT Imaging and its subsidiary prior to the Business Combination and to QT Imaging Holdings and its subsidiaries after the Business Combination. Terms not defined herein are as defined in the annual report.
Overview
We are a medical device company founded in 2012 and engaged in the research, development, and commercialization of innovative body imaging systems using low energy sound. We believe that medical imaging is critical to the detection, diagnosis, and treatment of disease and that it should be safe, affordable, and accessible. Our goal is to improve global health outcomes through the development and commercialization of imaging devices that address critical healthcare challenges with accuracy and precision.
101
With the support of nearly $18 million in financial support from the U.S. National Institutes of Health, we developed a novel, comprehensive body imaging technology that has high resolution, high sensitivity, high specificity, high positive and negative predictive values, and is safe and inexpensive. The technology is based on ultra-low frequency transmitted sound and uses a one-of-a-kind novel sound back-scatter design and inverse-scattering reconstruction to create its images.
Our current QT Breast Scanner is a Class II device subject to premarket notification and clearance under Section 510(k) of the FDCA. On August 23, 2016, we (formerly, QT Ultrasound LLC) submitted a Section 510(K) Summary of Safety and Effectiveness application for the QT Breast Scanner in accordance with 21 CFR 807.92 under 510(K) Number K162372. As part of meeting the general requirements for basic safety and essential performance of the QT Breast Scanner (formerly, QT Ultrasound Breast Scanner) pursuant to AAMI ES60601-1:2005/(R)2012 and A1:2012 Medical electrical equipment, testing was conducted by Intertek, an independent testing laboratory, located in Menlo Park, CA. Intertek also conducted applicable testing pursuant to IEC 60601-1-6 Edition 3.1 2013-10-Medical electrical equipment Part 1-6 General requirements for safety - Collateral Standard: Usability.
In addition, we conducted, and Intertek witnessed, all applicable testing pertaining to the requirements for the safety of ultrasonic medical diagnostic and monitoring equipment and to demonstrate compliance with the “Acoustic Output Measurement Standard for Diagnostic Ultrasound Equipment”. This test on acoustic output was pursuant to IEC 60601-2-37 Edition 2.0.2007 Medical electrical equipment - Part 2-37: Particular requirements for the basic safety and essential performance of ultrasonic medical diagnostic and monitoring equipment. Finally, system verification testing was conducted to ensure that the QT Breast Scanner met all design and other requirements including but not limited to that no new issues of safety or effectiveness compared to the predicate device, SoftVue System manufactured by Delphinus Medical Technologies, were raised.
Since our inception, we have devoted substantially all our financial resources to acquiring and developing the base technology for our body imaging systems, conducting research and development activities, securing related intellectual property rights, and for general corporate operations and growth. On June 6, 2017, FDA, in response to QT Imaging’s Section 510(K) Summary of Safety and Effectiveness premarket notification, determined that the QT Breast Scanner is substantially equivalent to the predicate device. Our use of the words “safe”, “safety”, “effectiveness”, and “efficacy” in relation to the QT Breast Scanner in this Management's Discussion and Analysis and all other documents related to us is limited to the context of the Section 510(K) Summary of Safety and Effectiveness that was reviewed and responded to by the FDA.
Our strategies for commercializing the QT Breast Scanner include the following:
•Create disruptive technological innovation (software, artificial intelligence, and smart physics) to improve medical imaging and thus health care quality and access.
•Continue to improve our high quality, high resolution, native 3D, reproducible image quality regardless of operator or breast size/tissue type breast imaging technology, as well as the techniques for quantifiable analysis, comparison, and training.
•Partner with strategic business and distribution channels to address the U.S. market for breast imaging immediately and, other regions in the future, to place the QT Breast Scanner in hospitals, radiology centers, etc. and generate awareness of the benefits of our technology.
•Perform small scale manufacturing internally to the Company and partner strategically for large scale manufacturing.
•Expand the market by supporting additional Direct-to-Customer and Direct-to-Patient approaches to enable the ability to lower health care costs and increase access via personal medical imaging.
•Provide a new social and economic opportunity for consumers to take control of some aspects of their own health care—such as imaging for minor injuries or medical conditions without needing a healthcare “gate-keeper.”
•Focus our intellectual capabilities and ethical framework to become unified in our mission to improve the quality and lower the cost of health care world-wide . . . “It’s about time.”
Consistent with our strategy, on May 31, 2023, we entered into the NXC Sales Agent Agreement with NXC, pursuant to which we appointed NXC as the non-exclusive agent for the sale of QT Imaging products and services in non-exclusive territories: the U.S., U.S. territories, and U.S. Department of Defense installations. Additionally, NXC was appointed as the exclusive servicer of QT Imaging products sold by NXC under the terms of the NXC Agreement. Effective June 10, 2024,
102
the NXC Agreement was superseded by the NXC Distribution Agreement that was entered into with NXC on June 18, 2024. Under the NXC Distribution Agreement, NXC is appointed as the exclusive reseller to market, advertise, and resell QT Breast Scanners in the U.S. and U.S. territories. NXC will purchase for the purpose of reselling, leasing or renting QT Breast Scanners directly to its customers, but is not obligated to purchase any particular quantity of QT Breast Scanners from us. We have reserved the right to sell directly to customers as an exception. Furthermore, we may, in our sole discretion, sell the QT Breast Scanners to any other person or entity anywhere in the world without notice to NXC or NXC’s prior consent. NXC is also allowed to assign sales agents for the purpose of QT Breast Scanner sales. NXC’s purchases will be in accordance with an agreed upon product pricing schedule (subject to change upon 60 days’ prior written notice by us), provided that neither NXC nor its assigned sales agents may mark-up the cost of the QT Breast Scanners more than twenty percent (20%) unless otherwise mutually agreed to between NXC and us. Each order will include information reasonably requested by us and is subject to our acceptance, after which it becomes an approved order. Any such approved orders are non-cancellable and not subject to rescheduling after acceptance by us. Any orders not accepted by us in writing are deemed rejected. As of December 31, 2024, we have delivered eight QT Breast Scanners to NXC’s customers pursuant to the NXC Sales Agent Agreement and NXC Distribution Agreement. On December 11, 2024, we and NXC entered into the Amended Distribution Agreement, which amends and restates the NXC Distribution Agreement in its entirety, making some modifications to the NXC Distribution Agreement but retaining other terms. We further amended the Amended Distribution Agreement on March 28, 2025. The Amended Distribution Agreement has a term that runs until December 31, 2026, unless earlier terminated or extended by mutual written agreement.
We have also entered into the Canon Letter of Intent with CMSU and CMSC pursuant to which CMSC purchased and acquired two QT Breast Scanners in the first half of 2024. The Canon Letter of Intent provided that CMSC would conduct, and pursuant to the Feasibility Study Agreement, CMSC conducted, the Feasibility Study on the QT Breast Scanners that it acquired, including product quality validation, development and manufacturing studies, clinical evaluation, regulatory investigation and marketing validation. The Feasibility Study was completed in the second half of 2024.
The Canon Letter of Intent provided that upon successful conclusion of the Feasibility Study, we and CMSC intended to engage in a good faith discussion to develop a binding OEM manufacturing agreement with CMSC.
On March 28, 2024, we entered into the Feasibility Study Agreement with CMSC. The term of the Feasibility Study Agreement commenced on March 28, 2024 and remained in force until the end of December 2024. In connection with the Feasibility Study Agreement, CMSC initiated studies to evaluate the business, technical, and clinical values of our ultrasound QT Breast Scanner including product quality validation, development and manufacturing studies, clinical evaluation, regulatory investigation, and market validation. CMSC has no right to reverse engineer the QT Breast Scanner and may only modify and disassemble the QT Breast Scanner as necessary to conduct the Feasibility Study.
On March 28, 2025, we entered into the Canon Manufacturing Agreement with CMSC. Pursuant to the terms of the Canon Manufacturing Agreement, we appoint CMSC as the exclusive manufacture of the QT Breast Scanners to be distributed by NXC. The purchase prices applicable to the purchase orders as of the date of the Canon Manufacturing Agreement shall be separately agreed between the parties in writing. The term of the Canon Manufacturing Agreement is through December 31, 2026.
We have incurred net operating losses and negative cash flows from operations since our inception and had an accumulated deficit of $31,940,527 as of December 31, 2024. During the year ended December 31, 2024, we incurred a net loss of $8,984,880 and used $10,033,477 of cash in operating activities, which includes the repayment of net liabilities assumed from the business combination. We continue to incur losses, and our ability to achieve and sustain profitability will depend on the achievement of sufficient revenues to support our cost structure. We may never achieve profitability and, unless and until we do, we will need to continue to raise additional capital.
We expect to incur additional recurring administrative expenses associated as a publicly traded company, including costs associated with compliance under the Exchange Act, annual and quarterly reports to stockholders, transfer agent fees, audit fees, incremental director and officer liability insurance costs, Sarbanes-Oxley Act compliance readiness, and director and officer compensation.
Recent Developments
On November 15, 2023, we entered into the SEPA with GigCapital5 and Yorkville, pursuant to which, upon the Closing of the Business Combination, we can sell to Yorkville up to $50.0 million of our common stock at our request any time during the 36 months following the Closing of the Business Combination. In addition, pursuant to the SEPA, we were entitled to and did request a “Pre-Paid Advance” from Yorkville and issued Yorkville the Yorkville Note in the amount of $10.0 million at the closing of the Business Combination for such Pre-Paid Advance. As consideration for the Pre-Paid
103
Advance, immediately prior to, and substantially concurrently with, the closing of the Business Combination, we issued to Yorkville the Company Shares which were that number of QT Imaging shares which converted in the aggregate into 1,000,000 shares of our common stock upon the completion of the Business Combination. On March 4, 2024, we received the Pre-Paid Advance of $9,025,000 from Yorkville and issued Yorkville the Yorkville Note that was originally due 15 months from the date of issuance, and accrues interest on the outstanding balance of the Yorkville Note at an annual rate equal to 6%, subject to an increase to 18% upon an event of default as described in the Yorkville Note. The Yorkville Note is convertible by Yorkville into shares of our common stock.
On September 13, 2024, a “Trigger Event” occurred under the terms of the Yorkville Note that resulted in us making a payment of $1,521,581 to Yorkville, which comprised of $1,145,407 of principal, $318,904 of accrued interest, and $57,270 of 5% early payment premium. On September 26, 2024, we and Yorkville entered into the Omnibus Amendment, pursuant to which we and Yorkville agreed to amend certain terms of the Yorkville Note to reduce the our obligations resulting from the occurrence of the Trigger Event. Pursuant to the Omnibus Amendment, the maturity date of the Yorkville Note was extended approximately six months from June 4, 2025 to December 15, 2025. Further, the Omnibus Amendment acknowledged our obligation to make monthly payments to Yorkville in the amount of the Trigger Principal Amount due to the occurrence of the Trigger Event and revised the Yorkville Note to provide that no further monthly payments will be owed during the period beginning on the date of the Omnibus Amendment and ending on January 15, 2025. In exchange for this relief, beginning on January 15, 2025, and continuing on the same day of each successive calendar month until and including November 15, 2025, whether or not a Trigger Event has occurred and is continuing as of such dates, we agreed to make monthly payments in an amount equal to $500,000 of principal plus the payment premium of 5% and accrued and unpaid interest under the Yorkville Note as of each payment date. Such monthly payments under the Omnibus Amendment were not to be reduced or offset by any amount, including, but not limited to, any net sales proceeds of the Company Shares or any value of the Company Shares based on the volume-weighted average price (“VWAP”) as quoted by Bloomberg, LP. The Omnibus Amendment also provided that 100% of the proceeds of the sale of the remaining 400,000 Company Shares held at the time of entry into the Omnibus Amendment by Yorkville shall be retained by Yorkville and shall not be used to offset or reduce any amounts owed under the Yorkville Note, as amended by the Omnibus Amendment, or to otherwise benefit us in any way. The Omnibus Amendment also provided that in the event that the common stock is delisted from trading on the Nasdaq Stock Market, Yorkville consents to the occurrence of such delisting from the Nasdaq Stock Market, if it is to happen, and that it will not constitute an Event of Default as per the Omnibus Amendment, provided that (i) we us our best efforts to have the common stock relisted on The Nasdaq Capital Market as soon as possible and (ii) Company’s common stock is listed on the OTC Markets’ OTCQX market tier within 30 days in the event that a delisting from the Nasdaq Stock Market occurs.
On October 31, 2024, we and Yorkville executed the Second Amendment, pursuant to which the maturity date of the Yorkville Note was extended from December 15, 2025 to March 31, 2026. Further, the Second Amendment acknowledged our obligation to make monthly payments to Yorkville in the amount of the Trigger Principal Amount due to the occurrence of the Trigger Event and no further monthly payments will be owed during the period beginning on the date of the Second Amendment and ending on February 15, 2025. In exchange for this relief, beginning on February 15, 2025, and continuing on the same day of each successive calendar month until and including February 15, 2026, whether or not a Trigger Event has occurred and is continuing as of such dates, we agreed to make monthly payments in an amount equal to $500,000 plus the payment premium plus accrued and unpaid interest as of each such payment date. Such monthly payments under the Second Amendment were not to be reduced or offset by any amount, including, but not limited to, any net sales proceeds of the Company Shares or any value of the Company Shares based on the VWAP as quoted by Bloomberg, LP. Further, pursuant to the terms of the Second Amendment, we elected to reduce the Floor Price to $0.50 per share, effective as of the date of the Second Amendment. The Second Amendment also provides that in the event that the common stock is delisted from trading on the Nasdaq Stock Market, Yorkville consents to the occurrence of such delisting from the Nasdaq Stock Market, if it is to happen, and that it will not constitute an Event of Default as defined per the Omnibus Amendment, provided that (i) we use our best efforts to have the common stock relisted on the Nasdaq Stock Market as soon as possible and (ii) the common stock is listed on the OTC Markets’ OTCQX or OTCQB market tiers within 30 days in the event that a delisting from the Nasdaq Stock Market occurs.
On November 4, 2024, Yorkville converted $254,593 of outstanding principal into 384,059 shares of common stock with an applicable conversion price of $0.6629 per share. The principal balance of the Yorkville Note was $8,600,000 following the November 4, 2024 conversion notice received from Yorkville. On December 6, 2024, Yorkville converted an additional $259,588 of outstanding principal under the Yorkville Note into 519,177 shares of common stock with an applicable conversion price of $0.50 per share. The principal balance of the Yorkville note was $8,340,411 following the December 6, 2024 conversion notice received from Yorkville.
104
On November 12, 2024, we and certain related parties entered into the Securities Purchase Agreement for the issuance of shares of common stock plus warrants for the purchase of common stock with an aggregate purchase price of $2,560,000 in exchange for 4,383,558 shares of common stock at an issuance price of $0.584 per share and 4,383,558 warrants with an exercise price of $0.672 per share.
On January 9, 2025, we and Yorkville entered into the Third Amendment, pursuant to which, we and Yorkville agreed that for $1.5 million of the then current outstanding balance due under the Yorkville Note (principal and unpaid accrued interest), the Fixed Price for conversion shall be modified to $0.584 per share, and for the remainder of the balance, the Fixed Price shall not be changed but shall remain $4.61395 per share as provided for in the Yorkville Note when we issued it on March 4, 2024. Further, the Third Amendment removed our obligation to make monthly payments to Yorkville, previously owing due to the occurrence of the Trigger Event, such that no further monthly payments will be owed during the period beginning on the date of the Third Amendment and ending on the maturity date of the Yorkville Note of March 31, 2026. In exchange for this relief, the aggregate purchase price owed to us from the first Advance that occurs pursuant to the terms of the SEPA shall be paid by Yorkville offsetting the amount of the Advance Proceeds against an equal amount outstanding under the Yorkville Note (first towards accrued and unpaid interest, and then towards outstanding principal and the corresponding payment premium in respect of such principal amount, if applicable), and that for any subsequent Advances pursuant to the terms of the SEPA, Yorkville shall pay half of such Advance Proceeds directly to us and the other half of such Advance Proceeds shall be paid by Yorkville offsetting the amount of the Advance Proceeds against an equal amount outstanding under the Yorkville Note (first towards accrued and unpaid interest, and then towards outstanding principal and the corresponding payment premium in respect of such principal amount, if applicable). On January 9, 2025, we delivered our first Advance Notice under the SEPA for the sale of 885,000 shares of common stock. This resulted in the reduction of an additional $182,682 in principal of the Yorkville Note.
On January 9, 2025, we also entered into the Cable Car Amendment with Cable Car to amend certain terms of the Cable Car Note, including a reduction of the conversion price for the Cable Car Note to $0.584 per share. Further, the Cable Car Amendment provides that the maturity date for the Cable Car Note shall be extended to March 31, 2026, in consideration for which, the Company shall pay a fee of $90,000 to Cable Car for the extension, with such fee being added to the amount due and payable on such maturity date, unless the Cable Car Note is earlier converted pursuant to its terms, in which event the Extension Fee shall also be converted. No interest shall accrue or be due on the Extension Fee. Pursuant to the Cable Car Amendment, interest shall accrue on the outstanding principal balance of the Cable Car Note at an annual rate equal to 6%, with interest being calculated based on a 365-day year and the actual number of days elapsed, to the extent permitted by applicable law. Interest shall be due and payable on the maturity date for the Cable Car Note, unless the Cable Car Note is earlier converted pursuant to its terms, in which event such accrued and unpaid interest shall also be converted.
On February 26, 2025, the Company entered into a credit agreement (the “Credit Agreement”) that provides a senior secured term loan (the “Lynrock Lake Term Loan”) with Lynrock Lake. The Credit Agreement is secured by a first priority lien on substantially all assets of the Company and provides for a term loan in the aggregate principal amount of $10,100,000 at an interest rate of 10.0% per annum, compounded quarterly. The maturity date of the Credit Agreement is March 31, 2027. Furthermore, in connection with the Lynrock Lake Term Loan, the Company issued to Lynrock Lake, pursuant to the terms of a Warrant to Purchase common stock (the “Lynrock Lake Warrant”), warrants to purchase 61,000,000 shares of its common stock at an exercise price of $0.40 per share. The Lynrock Lake Warrant is exercisable until February 26, 2035. Lynrock Lake may cashless exercise the Lynrock Lake Warrant. The Lynrock Lake Warrant is also subject to anti-dilution adjustments to the exercise price and the number of shares which may be purchased upon exercise of the Lynrock Lake Warrant in the event that the Company issues shares of common stock (or derivative securities) at a price that is either less than the $0.40 exercise price or the fair market value of a share of common stock from the immediately prior trading day.
On February 26, 2025, the Company used a portion of the proceeds of the Lynrock Lake Term Loan to pay Yorkville an amount equal to $3,000,000 in cash and issued to Yorkville warrants to purchase 15,000,000 shares of its common stock at an exercise price of $0.40 per share pursuant to a Warrant to Purchase common stock (the “Yorkville Warrant”) to fully settle and discharge the Company’s obligations under the Yorkville Note and extinguish the Yorkville Note as having been fully performed. The Yorkville Warrant is exercisable until February 26, 2030. Yorkville may cashless exercise the Yorkville Warrant. The Yorkville Warrant is also subject to adjustments in the event that the Company’s common stock undergoes a split, reverse-split or similar event. Furthermore, the Yorkville Warrant has provided the holder with piggyback registration rights. The Company and Yorkville also entered into that certain Termination Agreement, dated February 26, 2025 (the “Termination Agreement”), pursuant to which the parties acknowledged the termination of the SEPA effective February 26, 2025.
105
On February 26, 2025, the Company used a portion of the proceeds of the Lynrock Lake Term Loan to pay Cable Car an amount equal to the full principal, interest and fees amount of approximately $1,625,000 in cash to fully settle and discharge the Company’s obligations under the Cable Car Note and extinguish the Cable Car Note as having been fully performed.
Components of Our Results of Operations
Revenue
Revenue consists of revenue from the sale of our products including the QT Breast Scanner, accessories, and related services, which are primarily training and maintenance. For sales of products (which include the QT Breast Scanner and any accessories), revenue is recognized when a customer obtains control of the promised goods. The amount of revenue recognized reflects the consideration we expect to be entitled to receive in exchange for these goods. Service revenue is generally related to maintenance and training the customer.. Service revenue is recognized at the time the related performance obligation is satisfied, in an amount that reflects the consideration that we expect to receive in exchange for those services.
Cost of Revenue
Cost of revenue consists of our product costs, including manufacturing costs, personnel costs and benefits, duties and other applicable importing costs, shipping and handling costs, packaging, warranty replacement costs, fulfillment costs and inventory obsolescence and write-offs. We expect our cost of revenue to increase in absolute dollars and decrease as a percentage of revenues over time as we shift to new manufacturing processes and vendors that we anticipate will result in greater efficiency and lower per unit costs.
We expect we will continue to invest additional resources into our products to expand and further develop our offerings. The level and timing of investment in these areas could affect our cost of revenue in the future.
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred in connection with the research and development of our products, which include payroll and payroll related expenses, facilities costs, depreciation expense, materials and supplies, and consultant costs.
We expense all research and development costs in the periods in which such costs are incurred. Research and development activities are central to our business. We expect that our research and development expenses will increase substantially for the foreseeable future as we continue to invest in the development of the QT Breast Scanner and devote significant resources to the research and development of the full-body scanner product candidate intended for orthopedic and pediatric use.
We cannot reasonably determine the nature, timing and costs of the efforts that will be necessary to complete the enhancements of the QT Breast Scanner, or estimate the nature, timing and costs that will be necessary to complete the development of, and obtain regulatory approval for, the full-body scanner product candidate. The process of conducting the necessary research and development to obtain regulatory approval of a product candidate is costly and time-consuming, and the successful development of our product candidates is highly uncertain. Our research and development expenses may vary significantly based on factors such as, without limitation:
•The timing and progress of development activities;
•Our ability to maintain our current research and development programs and to establish new ones;
•The receipt of regulatory approvals from applicable regulatory authorities without the need for independent clinical trials or validation;
•Duration of subject participation in any trials and follow-ups;
•The countries and jurisdictions in which the trials are conducted;
•Length of time required to enroll eligible subjects and initiate trials;
•Per trial subject costs;
106
•Number of trials required for regulatory approval;
•The timing, receipt, and terms of any marketing approvals from applicable regulatory authorities;
•The success of our distribution arrangements, and our ability to establish new licensing or collaboration arrangements;
•Establishing contract manufacturing partnerships or making arrangements with third-party manufacturers;
•The hiring and retention of research and development personnel;
•Obtaining, maintaining, defending, and enforcing intellectual property rights; and
•The phases of development of our product candidates.
Any changes in the outcome of any of these variables with respect to the development of our products or product candidates could significantly change the costs and timing associated with the development of these products and product candidates.
Selling, General and Administrative Expenses
Selling, general and administrative expenses consist primarily of personnel costs, costs related to maintenance and filings of intellectual property, and other expenses for outside professional services, including legal, consulting, investor relations, audit and accounting services. Our personnel costs consist of salaries, benefits and stock-based compensation expenses. Selling, general and administrative expenses include facilities, depreciation and other expenses, which include direct or allocated expenses for rent and maintenance of facilities and insurance. Selling, general and administrative expenses also include consulting expenses and costs for conferences, meetings, and other events.
We anticipate that our selling, general and administrative expenses will increase to support our expanding headcount and operations, increased costs of operating as a public company, the development of a commercial infrastructure to support commercialization of our products and product candidates, increased support for existing and new distribution partner relationships, and the use of outside service providers such as insurers, consultants, lawyers, and accountants. We also expect selling expenses to increase in the near term as we promote our brand through marketing and advertising initiatives, expand market presence and hire additional personnel to drive penetration and generate leads.
Results of Operations
Comparison of the years ended December 31, 2024 and 2023
Year Ended December 31, | Change | ||||||||||||||||||||||
| 2024 | 2023 | $ | % | ||||||||||||||||||||
| Revenue | $ | 4,878,665 | $ | 40,355 | $ | 4,838,310 | N.M. | ||||||||||||||||
| Cost of revenue | 2,238,820 | 134,988 | 2,103,832 | N.M. | |||||||||||||||||||
| Gross profit (loss) | 2,639,845 | (94,633) | 2,734,478 | N.M. | |||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||
| Research and development | 3,267,340 | 1,485,636 | 1,781,704 | 120 | % | ||||||||||||||||||
| Selling, general and administrative | 11,549,512 | 3,427,690 | 8,121,822 | 237 | % | ||||||||||||||||||
| Total operating expenses | 14,816,852 | 4,913,326 | 9,903,526 | 202 | % | ||||||||||||||||||
| Loss from operations | (12,177,007) | (5,007,959) | (7,169,048) | (143) | % | ||||||||||||||||||
Other expense, net | (560,648) | (544,566) | (16,082) | (3) | % | ||||||||||||||||||
| Change in fair value of warrant liability | 187,173 | — | 187,173 | 100 | % | ||||||||||||||||||
| Change in fair value of derivative liability | 4,817,600 | — | 4,817,600 | 100 | % | ||||||||||||||||||
| Change in fair value of earnout liability | 3,230,000 | — | 3,230,000 | 100 | % | ||||||||||||||||||
| Interest expense, net | (4,497,781) | (544,826) | (3,952,955) | (726) | % | ||||||||||||||||||
Loss before income tax expense (benefit) | (9,000,663) | (6,097,351) | (2,903,312) | (48) | % | ||||||||||||||||||
Income tax expense (benefit) | (15,783) | 1,600 | (17,383) | N.M. | |||||||||||||||||||
| Net loss and comprehensive loss | $ | (8,984,880) | $ | (6,098,951) | $ | (2,885,929) | (47) | % | |||||||||||||||
107
N.M. - Not meaningful
Revenue
Revenue increased by $4,838,310 to $4,878,665 for the year ended December 31, 2024 from $40,355 for the year ended December 31, 2023. The increase in revenue was primarily attributable to the sale of eleven QT Breast Scanners in the year of 2024 as compared with no scanners sold in the year of 2023 due to the timing of sales orders received, availability of scanners that were earmarked and ready for sale to customers.
Cost of Revenue
Cost of revenue increased by $2,103,832 to $2,238,820 for the year ended December 31, 2024 from $134,988 for the year ended December 31, 2023. The increase in cost of revenue was primarily attributable to the sale of eleven QT Breast Scanners in the year of 2024 as compared with no scanners sold in the year of 2023, which was partially offset by inventory write-offs in the year of 2023.
Operating Expenses
Research and Development Expenses
Research and development expenses increased by $1,781,704 to $3,267,340 for the year ended December 31, 2024 from $1,485,636 for the year ended December 31, 2023. The increase in research and development expenses was primarily attributable to an increase in employee compensation costs of $1,120,308 and professional and outside services costs of $491,368, and decrease in grant income from the National Institute of Health of $260,518, which was partially offset by a decrease in depreciation and amortization expense of $229,007.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased by $8,121,822 to $11,549,512 for the year ended December 31, 2024 from $3,427,690 for the year ended December 31, 2023. This change was primarily attributable to increases in non-recurring transaction expenses of $3,944,924 related to the business combination, professional and outside services costs of $723,655, non-recurring recruiting and employee conversion costs of $210,500, employee compensation costs of $1,734,342, insurance costs of $776,185, and information technology costs of $276,405.
Other expense, net
Other expense, net increased by $16,082 to $560,648 for the year ended December 31, 2024, from $544,566 for the year ended December 31, 2023. The expense for the year ended December 31, 2024 was primarily due to a loss on debt extinguishment of $383,511 related to the conversion of the Extension Note, and a modification expense of $200,513 related to the decrease in exercise price of our private placement warrants and working capital note warrants. The expense for the year ended December 31, 2023 was primarily due to a debt extinguishment loss of $376,086 related to an amendment and issuance of the senior secured convertible promissory note to US Capital as part of the Bridge Loan (as defined below), and an induced conversion expense of $168,356 related to the conversion of the principal balance and accrued interest of the 2020 Notes into 100,000 shares of QT Imaging common stock during the year ended December 31, 2023.
Change in fair value of warrant liability
Change in fair value of warrant liability was $187,173 for the year ended December 31, 2024. The change in fair value of warrants relates to the liability classified private placement warrants and working capital note warrants and reflects the decrease of the publicly traded price per warrant during the year ended December 31, 2024.
Change in fair value of derivative liability
Change in the fair value of derivative liability was $4,817,600 for the year ended December 31, 2024. The change in fair value of derivatives was primarily driven by the decline in the value of our common stock during the year ended December 31, 2024.
Change in fair value of earnout liability
Change in the fair value of earnout liability was $3,230,000 for the year ended December 31, 2024. The change in fair value of derivatives was primarily driven by the decline in the value of our common stock during the year ended
108
December 31, 2024. The earnout liability relates to the contingent consideration for the Merger Earnout Consideration Shares pursuant to the Business Combination Agreement dated December 8, 2022, as amended in September of 2023. We did not have an earnout liability for the year ended December 31, 2023.
Interest expense, net
Interest expense, net increased by $3,952,955 to $4,497,781 for the year ended December 31, 2024 from $544,826 for the year ended December 31, 2023. This change is primarily driven by an increase in the amortization of debt discount of $3,568,137 for the Bridge Loans, the Pre-Paid Advance, the Cable Car Note and the Extension Note, an increase in interest expense of $474,106 and payment premium of $57,270 for the Pre-Paid Advance, and an increase in interest of $160,000 paid in cash related to the Bridge Loans, partially offset by decrease in interest expense of $399,398 related to a convertible promissory note with US Capital (the “US Capital Note”).
Liquidity and Capital Resources
Sources of Liquidity
Liquidity describes our ability to meet financial obligations which arise during the normal course of business. To date, we have financed our operations primarily through the sale of equity securities, issuances of convertible notes and other debt, and grants from the U.S. government. We expect to derive future liquidity primarily through our revenues with customers and sale of equity securities. Our current liquidity position consists of cash on hand and certificates of deposit.
Since our inception, we have incurred significant operating losses and negative cash flows. As of December 31, 2024 and 2023, we had an accumulated deficit of $31,940,527 and $17,770,145, respectively. As of December 31, 2024 and 2023, we had cash and restricted cash and cash equivalents of $1,192,104 and $184,686, respectively. Our primary uses of cash are for general working capital requirements, and capital expenditures. Cash flows from operations have been historically negative as we invested in product development, clinical trials, and manufacturing. We expect to be cash flow negative for the foreseeable future, although we may have quarterly results where cash flows from operations are positive.
In connection with the Business Combination, we entered into various agreements to obtain financing through the issuance of debt and through stock subscription agreements. In March of 2024, we received the Pre-Paid Advance net of issuance costs of $9,025,000 from Yorkville pursuant to the SEPA and issued Yorkville the Yorkville Note in the amount of $10.0 million for such Pre-Paid Advance, $500,000 of cash proceeds from an investor related to a stock subscription agreement, and $1,500,000 in cash proceeds through a note payable from Cable Car. The SEPA provides us with access to an additional $40 million of potential capital through the issuance of common stock to Yorkville. During the time we have a balance under the Yorkville Note, advances can be received under the SEPA with written consent of Yorkville or upon a Trigger Event, which following the effectiveness of the registration statement on Form S-1 that we filed to register the shares to be issued pursuant to the SEPA occurs when the daily VWAP is less than the Floor Price (as such term is defined in the Yorkville Note) for five consecutive trading days, which prior to October 31, 2024, was $0.8768 per share. As previously disclosed in a Current Report on Form 8-K with the SEC on September 13, 2024, a Trigger Event occurred on September 11, 2024, following which on September 13, 2024, we made a payment to Yorkville on the Yorkville Note of $1,521,581 which included $1,145,407 as repayment of principal. See Yorkville Pre-Paid Advance below for a further discussion of the effect of this Trigger Event and an amendment to the documents pertaining to the Yorkville Note. On November 12, 2024, the Company executed a securities purchase agreement with related parties for the issuance of shares of common stock plus warrants for the purchase of common stock as a Private Investment in Public Equity (the “PIPE”) with an aggregate purchase price of $2.56 million, the closing of which occurred on November 22, 2024. On December 11, 2024, the Company and NXC entered into the Amended Distribution Agreement (which we further amended on March 28, 2025), which provides the Company with MOQs that could result in cash inflows of up to $18.0 million in 2025 and $27.0 million in 2026. On February 26, 2025, the Company entered into the Credit Agreement that provides the Lynrock Lake Term Loan with Lynrock Lake for a term loan in the aggregate principal amount of $10.1 million and repaid the secured Cable Car Note, and fully settled its obligations under the Yorkville Note and terminated the Yorkville SEPA by paying $3.0 million in cash and issuing a 5-year warrant for 15 million shares. Net of these payments, the Company had $5.4 million of net proceeds for working capital purposes. Management believes that the cash received for the Lynrock Lake Term Loan and additional revenue anticipated from MOQs per the Amended Distribution Agreement will be sufficient to fund the Company’s current operating plan for at least the next 12 months.
Our future capital requirements will depend on many factors, including our growth rate, the timing and extent of our spending to support research and development activities, the timing and cost of establishing additional sales and marketing capabilities, and the timing and cost to introduce new and enhanced products. In the event that additional financing is required from outside sources, we may not be able to raise it on terms acceptable to us or at all. Any additional debt
109
financing obtained by us in the future could also involve restrictive covenants relating to our capital-raising activities and other financial and operational matters, which may make it more difficult for us to obtain additional capital and to pursue business opportunities, including potential acquisitions. Additionally, if we raise additional funds through further issuances of equity, convertible debt securities or other securities convertible into equity, our existing stockholders could suffer significant dilution in their percentage ownership of the Company, and any new equity securities we issue could have rights, preferences and privileges senior to those of holders of our common stock. If we are unable to obtain adequate financing or financing on terms satisfactory to us when we require it, our ability to continue to grow or support our business and to respond to business challenges could be significantly limited.
Paycheck Protection Program Loan
On February 24, 2021 and May 5, 2020, we received loans (“PPP Loans”) from US Bank to fund payroll, rent and utilities through the Paycheck Protection Program (“PPP”). We received partial forgiveness on the PPP Loans during fiscal year 2021. The remaining balances on the PPP Loans are being repaid on a monthly basis, with interest of 1% per annum and the final payment due in February 2026.
As of December 31, 2024, the total principal outstanding under the PPP Loans was $95,981, of which $86,784 was current and $9,197 was noncurrent. As of December 31, 2023, the total principal outstanding under the PPP Loans was $226,348, of which $130,366 was current and $95,982 was noncurrent.
Convertible Notes Payable
In June 2021, we entered into a convertible promissory note agreement (the “Note”) with USCG for advances of up to $10,000,000. We could have made advances on the Note up to six months after the inception of the Note unless extensions for advances were mutually agreed between both parties. The Note bore interest at 12% per annum on any amounts drawn with a maturity date of July 6, 2024. The Note was collateralized by all our assets and was guaranteed by QT Labs. The terms of the Note include non-financial covenants and, as of March 4, 2024 when the Note converted, we were in compliance with those covenants. Through December 31, 2023, we issued warrants in connection with the note to purchase a total of 5,091 shares of common stock which 3,540 shares were exercisable at a price of $12.40 per share and 1,551 shares were exercisable at a price of $11.67 per share. On March 4, 2024, these warrants were terminated in accordance with the Business Combination Agreement.
The Note was convertible, at our option, before the Note matured upon the closing of a single transaction or a series of transactions with a minimum of $15,000,000 of cash proceeds raised in the aggregate. If elected, the conversion price is 90% of the price per share in the qualified financing.
As of December 31, 2024, there was no amount outstanding for the Note and US Capital Note. As of December 31, 2023, the total Note and US Capital Note balance was $3,294,659 net of unamortized debt issuance costs of $36,194, and accrued interest of $50,037.
On March 4, 2024, the Note principal and related accrued interest balance of $3,233,388 and the US Capital Note principal balance of $200,000 (as further discussed below under the Bridge Loan section) was converted into 359,266 and 100,000 shares of our common stock, respectively. Additionally, warrants to purchase 16,320 shares of our common stock were net settled into 5,594 shares of our common stock.
Bridge Loan
In November 2023, we entered into a Securities Purchase Agreement and raised the private secured “Bridge Loan” in the aggregate amount of $1,000,000 from five investors.
Each Bridge Loan of $200,000 bore no interest but had a cash option value at the date maturity of 120%, or $240,000, of the Bridge Loan at each Bridge Lender’s option. The maturity date was the closing date of the Business Combination as defined in Note 1 to the accompanying consolidated financial statements. The Bridge Loan conversion was at $2.00 per share on a post-business combination. On March 4, 2024, 4 of the 5 Bridge Loan holders elected the cash option and were paid an aggregate of $960,000 on the Merger Date.
As of December 31, 2024, there was no amount outstanding for the Bridge Loan. As of December 31, 2023, the outstanding amount of the Bridge Loan, excluding the US Capital Note, was $774,337, net of unamortized debt issuance costs of $25,663.
110
Yorkville Pre-Paid Advance
On March 4, 2024, we received the Pre-Paid Advance of $10,000,000 from Yorkville and issued Yorkville the Yorkville Note in the amount of $10.0 million for such Pre-Paid Advance that was originally due 15 months from the date of issuance, and interest shall accrue on the outstanding balance of the Yorkville Note at an annual rate equal to 6%, subject to an increase to 18% upon an event of default as described in the Yorkville Note. The Yorkville Note is convertible by Yorkville into shares of our common stock. As consideration for the Pre-Paid Advance, immediately prior to, and substantially concurrently with, the closing of the Business Combination, QT Imaging issued to Yorkville that number of QT Imaging shares which converted in the aggregate into 1,000,000 shares of our common stock upon the completion of the Business Combination.
On September 13, 2024, we made a payment of $1,521,581 to Yorkville, which comprised of $1,145,407 of principal, $318,904 of accrued interest, and $57,270 of 5% early payment premium as a result of a trigger event occurring under the terms of the Yorkville Note.
On September 26, 2024, we and Yorkville entered into the Omnibus Amendment, pursuant to which we and Yorkville agreed to amend certain terms of the Yorkville Note to reduce our obligations resulting from certain trigger events. Pursuant to the Omnibus Amendment, the maturity date of the Yorkville Note was extended approximately six months from June 4, 2025 to December 15, 2025. Further, the Omnibus Amendment acknowledges our obligation to make monthly payments to Yorkville due to the occurrence of certain trigger events provided that no further monthly payments will be owed during the period beginning on the date of the Omnibus Amendment and ending on January 15, 2025. In exchange for this relief, beginning on January 15, 2025, and continuing on the same day of each successive calendar month until and including November 15, 2025, whether or not trigger events have occurred, we will make a monthly payment in an amount equal to $500,000 of principal plus a payment premium of 5% and unpaid accrued interest as of each payment date.
On October 31, 2024, we and Yorkville executed the Second Amendment, pursuant to which the maturity date of the Yorkville Note was extended from December 15, 2025 to March 31, 2026. Further, the Second Amendment acknowledges our obligation to make monthly payments to Yorkville due to the occurrence of the certain trigger events and no further monthly payments will be owed during the period beginning on the date of the Second Amendment and ending on February 15, 2025. In exchange for this relief, beginning on February 15, 2025, and continuing on the same day of each successive calendar month until and including February 15, 2026, whether or not trigger events have occurred and are continuing as of such dates, we will make monthly payments in an amount equal to $500,000 of principal plus a payment premium of 5% and unpaid accrued interest as of each such payment date. Such monthly payments will not be reduced or offset by any amount, including, but not limited to, any net sales proceeds of the immediately prior to, and substantially concurrently with, the Closing of the Business Combination, or any value of such shares based on the VWAP as quoted by Bloomberg, LP. Further, pursuant to the terms of the Second Amendment, we elected to reduce the Floor Price to $0.50 per share, effective as of the date of the Second Amendment.
On November 4, 2024, Yorkville converted $254,593 of outstanding principal into 384,059 shares of common stock with an applicable conversion price of $0.6629 per share. On December 6, 2024, Yorkville converted $259,588 of outstanding principal under the Yorkville Note into 519,177 shares of common stock with an applicable conversion price of $0.50 per share. The principal balance of the Yorkville Note was $8,340,411 following the two conversion notices received from Yorkville.
As of December 31, 2024, the outstanding amount of the Yorkville Note was $3,532,591, net of the unamortized debt discount of $4,807,820 and accrued interest of $155,203.
On January 9, 2025, we and Yorkville entered into the Third Amendment, pursuant to which, we and Yorkville agreed that for $1.5 million of the then current outstanding balance due under the Yorkville Note (principal and unpaid accrued interest), the fixed price for conversion shall be modified to $0.584 per share, and for the remainder of the balance, the fixed price shall not be changed but shall remain $4.61395 per share as provided for in the Yorkville Note when we issued it on March 4, 2024. Further, the Third Amendment removed our obligation to make monthly payments to Yorkville, previously owing due to the occurrence of the Trigger Event, such that no further monthly payments will be owed during the period beginning on the date of the Third Amendment and ending on the maturity date of the Yorkville Note of March 31, 2026. In exchange for this relief, the aggregate purchase price owed to us from the first Advance that occurs pursuant to the terms of the SEPA shall be paid by Yorkville offsetting the amount of the Advance Proceeds against an equal amount outstanding under the Yorkville Note (first towards accrued and unpaid interest, and then towards outstanding principal and the corresponding payment premium in respect of such principal amount, if applicable), and that for any subsequent Advances pursuant to the terms of the SEPA, Yorkville shall pay half of such Advance Proceeds directly to us and the other half of such Advance Proceeds shall be paid by Yorkville offsetting the amount of the Advance Proceeds against an
111
equal amount outstanding under the Yorkville Note (first towards accrued and unpaid interest, and then towards outstanding principal and the corresponding payment premium in respect of such principal amount, if applicable). On January 9, 2025, we delivered our first Advance Notice under the SEPA for the sale of 885,000 shares of common stock. This resulted in the reduction of an additional $182,682 in principal of the Yorkville Note.
On February 26, 2025, the Company used a portion of the proceeds of the Lynrock Lake Term Loan to pay Yorkville an amount equal to $3,000,000 in cash and issued to Yorkville warrants to purchase 15,000,000 shares of its common stock at an exercise price of $0.40 per share pursuant to the Yorkville Warrant to fully settle and discharge the Company’s obligations under the Yorkville Note and extinguish the Yorkville Note as having been fully performed. The Yorkville Warrant is exercisable until February 26, 2030. Yorkville may cashless exercise the Yorkville Warrant. The Yorkville Warrant is also subject to adjustments in the event that the Company’s common stock undergoes a split, reverse-split or similar event. Furthermore, the Yorkville Warrant has provided the holder with piggyback registration rights. The Company and Yorkville also entered into that certain Termination Agreement, dated February 26, 2025 (the “Termination Agreement”), pursuant to which the parties acknowledged the termination of the SEPA effective February 26, 2025.
Cable Car Loan
In February 2024, we and Cable Car entered into the Cable Car NPA, pursuant to which Cable Car agreed to advance $1,500,000 at the Closing of the Business Combination, as was evidenced by the Cable Car Note that may be convertible in certain circumstances into shares of the common stock at a conversion price of $2.00 per share, dated March 4, 2024. The Loan at issuance did not bear interest, and was originally due and payable 13 months after issuance, unless the time for payment is accelerated as a result of an event of default. As full compensation to Cable Car for the Cable Car Note to us in lieu of any simple or in-kind interest on the Cable Car Note, QT Imaging issued to Cable Car that number of QT Imaging shares of common stock which at the completion of the Business Combination would be converted in accordance with the terms of the Business Combination Agreement into 180,000 shares of our common stock.
As of December 31, 2024, the outstanding amount of the Cable Car Note was $1,366,458 net of issuance costs of $133,542.
On January 9, 2025, the Company and Cable Car entered into an Omnibus Amendment (the “Cable Car Amendment”) to amend certain terms of the Cable Car Note, including a reduction of the conversion price for the Cable Car Note to $0.584 per share. Further, the Cable Car Amendment provides that the maturity date for the Cable Car Note shall be extended to March 31, 2026, in consideration for which, the Company shall pay an extension fee (the “Extension Fee”) of $90,000 to Cable Car, with such fee being added to the amount due and payable on such maturity date, unless the Cable Car Note is earlier converted pursuant to its terms, in which event the Extension Fee shall also be converted. No interest shall accrue or be due on the Extension Fee. Pursuant to the Cable Car Amendment, interest shall accrue on the outstanding principal balance of the Cable Car Note at an annual rate equal to 6%, with interest being calculated based on a 365-day year and the actual number of days elapsed, to the extent permitted by applicable law. Interest shall be due and payable on the maturity date for the Cable Car Note, unless the Cable Car Note is earlier converted pursuant to its terms, in which event such accrued and unpaid interest shall also be converted. In addition, in connection with any sale, assignment, transfer, or other disposition (a “Cable Car Sale”) of any shares into which the Cable Car Note is converted pursuant to its terms, the Cable Car Amendment provides that to the extent such Sale is made pursuant to Rule 144, provided that Rule 144 is available as an exemption from the registration requirements for such Cable Car Sale, if requested by Cable Car and upon delivery by Cable Car of such customary representations and other documentation reasonably acceptable to the Company in connection with transactions relying upon Rule 144, the Company shall use commercially reasonable efforts to cause its transfer agent to remove any restrictive legends related to the book entry account holding such shares sold or disposed of by Cable Car without restrictive legends within two business days of such request.
On February 26, 2025, the Company used a portion of the proceeds of the Lynrock Lake Term Loan to pay Cable Car an amount equal to the full principal, interest and fees amount of approximately $1,625,000 in cash to fully settle and discharge the Company’s obligations under the Cable Car Note and extinguish the Cable Car Note as having been fully performed.
Related Party Convertible Notes Payable
In July 2020, we issued three convertible notes to three of its stockholders for advances up to $3,500,000 in principal (the “2020 Notes”) and bearing annual interest of 5% on any amounts drawn. An additional note was issued in March 2022 as part of the 2020 Notes, but with an annual interest rate of 8%. All principal and interest payments are due on or before July 1, 2025. The 2020 Notes are convertible, at the holder’s option, into shares of common stock at the lower of $14.59 per share or the offering price in a financing of at least $5,000,000 in equity from unaffiliated parties. As of December 31,
112
2024, an aggregate of 253,199 shares of common stock would be issued if the entire principal and interest under the 2020 Notes was converted.
As of December 31, 2024 and 2023, the outstanding amount of the 2020 Notes was $3,143,725 and accrued interest of $550,430 and $377,772, respectively.
In connection with the issuance of the Lynrock Lake Term Loan, on February 26, 2025, the maturity date on the these convertible notes was extended to October 21, 2027.
Related Party Working Capital Loan and Extension Note
On May 3, 2023, we issued a promissory note (the “Working Capital Note”) to a stockholder for a principal amount of $250,000. The Working Capital Note was subsequently amended and restated six times on June 12, 2023 to add an additional principal amount of $100,000, August 15, 2023 to add an additional principal amount of $75,000, August 29, 2023 to add an additional principal amount of $100,000, September 12, 2023 to add an additional principal amount of $75,000, September 15, 2023 to add an additional principal amount of $50,000, and October 26, 2023 to add an additional principal amount of $55,000, for an aggregate principal amount outstanding as of December 31, 2023 under the Working Capital Note of $705,000. The Working Capital Note was issued to provide us with additional working capital during the period prior to consummation of the Business Combination Agreement with GigCapital5. The Working Capital Note is interest-free and originally matured on the earlier of (i) the date on which we consummated the Business Combination with GigCapital5, Inc.; (ii) the date we wind up; or (iii) December 31, 2023. On March 4, 2024, the Working Capital Note was agreed to be amended and subordinated pursuant to and in accordance with the terms of the Business Combination Agreement. Effective on the Closing of the Business Combination, the Working Capital Note cannot be repaid prior to the repayment or conversion of the Yorkville Note issued to Yorkville. In connection with the issuance of the Lynrock Lake Term Loan, on February 26, 2025, the maturity date on the Related Party Working Capital Note Payable was extended to October 21, 2027.
On March 4, 2024, we assumed the $1,560,000 outstanding balance of the Extension Note from a related party and pursuant to the Business Combination Agreement. The Extension Note does not bear any interest and cannot be repaid prior to the repayment of the Pre-Paid Advance received from Yorkville. On November 12, 2024, the holder of the Extension Note entered into the Securities Purchase Agreement and surrendered the Extension Note on November 22, 2024 for cancellation in its entirety in exchange for the purchase of PIPE Shares and PIPE Warrants for the purchase of common stock with a purchase price of $1,560,000. On November 22, 2024, the Extension Note was cancelled in its entirety in exchange for the purchase of 2,671,232 shares of common stock at a purchase price of $0.584 per share and issuance of 2,671,232 of PIPE Warrants with exercise price of $0.672 per share.
Cash Flows
The following table provides information regarding our cash flows for the periods presented:
For Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
Net cash used in operating activities | $ | (10,033,477) | $ | (2,651,143) | ||||||||||
Net cash used in investing activities | (87,790) | (13,040) | ||||||||||||
Net cash provided by financing activities | 11,128,685 | 2,373,793 | ||||||||||||
Net increase (decrease) in cash and restricted cash and cash equivalents | $ | 1,007,418 | $ | (290,390) | ||||||||||
Net Cash Used In Operating Activities
Net cash used in operating activities was $10,033,477 for the year ended December 31, 2024 as compared to $2,651,143 for the year ended December 31, 2023. The primary use of our cash was to fund research and development and general and administrative expenses. Net cash used for the year ended December 31, 2024 consisted of a net loss of $8,984,880, adjusted for non-cash expenses primarily including depreciation and amortization of $230,804, stock-based compensation of $289,795, fair value of common stock issued in exchange for services and in connection with non-redemption agreements of $3,698,350, issuance of common stock in connection with a stock subscription agreement of $206,000, warrant modification expense of $200,513, loss on debt extinguishment of $383,511, non-cash interest of $3,589,728, decrease in warrant liability of $187,173, decrease in derivative liability of $4,817,600, decrease in earnout liability of $3,230,000, and the net change in operating assets and liabilities of $1,384,385. The net change in operating assets and liabilities was primarily due an increase accounts receivable of $67,119, an increase in prepaid expenses and
113
other current assets of $200,770, a decrease in accounts payable of $1,954,768, a decrease in accrued expenses and other current liabilities of $542,878, and a decrease in deferred revenue of $298,254, partially offset by a decrease in inventory of $1,506,746 and an increase in other liabilities of $172,658.
Net cash used for the year ended December 31, 2023 consisted of a net loss of $6,098,951, adjusted for non-cash expenses including depreciation and amortization of $480,694, stock-based compensation of $709,394, non-cash interest of $66,367, induced conversion expense of $168,356, debt extinguishment loss of $376,086, and non-cash operating lease expense of $8,246, partially offset by the net change in operating assets and liabilities of $1,655,033. The net change in operating assets and liabilities was primarily due to a decrease in inventory of $98,594, an increase in accounts payable of $876,074, an increase in accrued expenses and other current liabilities $645,840, and an increase in deferred revenue of $347,619, partially offset primarily by a decrease in other liabilities of $205,701 and an increase in prepaid expenses and other current assets of $116,103.
Net Cash used in Investing Activities
Net cash used in investing activities was $87,790 for the year ended December 31, 2024 as compared to $13,040 for the year ended December 31, 2023. The use of net cash used in investing activities for both periods was related to the purchase of property and equipment.
Net Cash provided by Financing Activities
During the year ended December 31, 2024, net cash provided by financing activities was $11,128,685, primarily due to $10,525,000 of net proceeds received from issuance of long-term debt related to the Yorkville Pre-Paid Advance and the Cable Car Note, net proceeds of $1,238,529 received from the Merger, net proceeds from sale of common stock and warrants of $999,998, and cash proceeds of $500,000 received from issuance of common stock pursuant to a subscription agreement, partially offset by repayment of the Bridge Loan of $800,000, cash paid for debt issuance costs of $59,069, and repayments against the Yorkville Note and PPP loans of $1,275,773.
During the year ended December 31, 2023, net cash provided by financing activities was $2,373,793, primarily due to $1,017,850 of net proceeds from the sale of QT Imaging common stock and QT Imaging warrants, proceeds of $800,000 from the Bridge Loan and $705,000 from the Working Capital Notes, partially offset by repayments against the PPP loans of $129,057 and cash paid to a lender for debt modification of $20,000.
Future Funding Requirements
We expect to incur increased significant expenses in connection with our ongoing activities, particularly as we continue the research and development of our products and product candidates, seek expanded regulatory clearances for the QT Breast Scanner, and build a U.S. sales and marketing team. As part of the effort to build the sales and marketing capabilities in the United States, QT Imaging entered into the Amended Distribution Agreement, pursuant to which QT Imaging appointed NXC as the exclusive agent for the sale of the QT Breast Scanner in the U.S. and U.S. territories. Since our consummation of the Merger, we expect to incur additional costs associated with operating as a public company. Our future funding requirements, both short-and long-term, will depend on many factors, including, without limitation:
•Having the cash to repay our debt obligations as they come due;
•Expand our current manufacturing operations and expand existing and build new partnerships with contract manufacturing third-party vendors;
•Purchase inventory for our planned shipments;
•Expand or enhance our distribution with third-party distribution channels;
•The progress and results of our trials and interpretation of those results by the FDA (and other regulatory authorities, as required);
•Seek regulatory clearances for product candidates and expanded regulatory clearance for the QT Breast Scanner;
•The cost of operating as a public company, including hiring additional personnel as well as increased director and officer insurance premiums, audit and legal fees, investor relations fees and expenses related to compliance with public company reporting requirements under the Exchange Act and rules implemented by the SEC and Nasdaq; and
114
•The costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending our intellectual property-related claims.
We plan to continue to incur substantial costs in order to conduct research and development activities necessary to develop a commercialized product. Additional capital will be needed to undertake these activities and commercialization efforts. We intend to raise such capital through the issuance of additional equity, borrowings and potential strategic alliances with other companies. If we raise additional capital through debt financing, we may be subject to covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. If such financing is not available at adequate levels or on acceptable terms, we could be required to significantly reduce operating expenses and delay, reduce the scope of or eliminate some of our development programs or our commercialization efforts, out-license intellectual property rights to our product candidates and sell unsecured assets, or a combination of the foregoing, any of which may have a material adverse effect on our business, results of operations, financial condition and/or our ability to fund our scheduled obligations on a timely basis, or at all.
Because of the numerous risks and uncertainties associated with manufacturing, research, development and commercialization of products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on, and could increase significantly as a result of, many factors, including, without limitation:
•The timing, receipt and amount of revenues from the sales of the QT Breast Scanner and related products and services, or any future approved or cleared products and product candidates, if any;
•The cost of future activities, including product sales, medical affairs, marketing, manufacturing and distribution for the QT Breast Scanner;
•The costs, timing, and outcomes of regulatory review of applications for expanded clearances for the QT Breast Scanner and clearance for other products;
•The scope, progress, results and costs of researching, developing and manufacturing our product candidates or any future product candidates, and conducting studies and clinical trials;
•The timing of, and the costs involved in, obtaining regulatory approvals or clearances for our product candidates or any future product candidates;
•The cost of manufacturing our product candidates or any future product candidates and any products we successfully commercialize, including costs associated with building out our manufacturing capabilities;
•The cost and time needed to attract and retain skilled personnel to support our continued growth;
•Our ability to establish and maintain strategic collaborations, licensing or other arrangements and the financial terms of any such agreements that we may enter into; and
•The costs associated with being a public company.
Additionally, our operating plans may change, and we may need additional funds to meet operational needs and capital requirements for future trials and other research and development activities. Until such time, if ever, as we can generate substantial product revenue, we expect to finance our cash needs through a combination of public or private equity offerings, debt financings, collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interest of our stockholders may be materially diluted, and the terms of such securities could include liquidation or other preferences that adversely affect the rights of our stockholders. Debt financing and preferred equity financing, if available, may involve agreements that include restrictive covenants that limit our ability to take specified actions, such as incurring additional debt, making capital expenditures or declaring dividends. In addition, debt financing would result in increased fixed payment obligations.
If we raise funds through collaborations, strategic partnerships or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or products, or grant licenses on terms that may not be favorable to us.
If we are unable to raise additional funds when needed, we may be required to delay, reduce, or eliminate our product development or future commercialization efforts, or grant rights to develop and market products that we would otherwise prefer to develop and market ourselves.
115
Our ability to continue as a going concern is dependent upon our ability to successfully accomplish these plans and secure sources of financing and attain profitable operations. If we are unable to obtain adequate capital, we could be forced to cease operations. See the section entitled “Risk Factors” for additional factors and risks associated with our capital requirements.
Off-Balance Sheet Arrangements
We did not have during the periods presented, and we do not currently have, any off-balance sheet arrangements.
Contractual Obligations
We lease our operating facilities in Novato, California, under a non-cancelable operating lease through May 31, 2027. There are no options or rights to extend the term of this lease.
Contingencies
Litigation
We are subject to occasional lawsuits, investigations and claims arising out of the normal course of business. As of the date the consolidated financial statements were available to be issued, management is not aware of any pending claims that will have a material impact on our consolidated financial statements.
Emerging Growth Company
We are an emerging growth company (“EGC”), as defined in the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (i) are no longer an emerging growth company, or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our consolidated financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
In addition, we intend to rely on the other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, if, as an EGC, we intend to rely on such exemptions, we are not required to, among other things: (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act; (ii) provide all of the compensation disclosure that may be required of non-emerging growth public companies under the Dodd- Frank Wall Street Reform and Consumer Protection Act; (iii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board (United States) regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); and (iv) disclose certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation.
We will remain an EGC under the JOBS Act until the earliest of (i) the last day of our first fiscal year following the fifth anniversary of the Closing of the Business Combination, (ii) the last date of our fiscal year in which we have total annual gross revenue of at least $1.235 billion, (iii) the date on which we are deemed to be a “large accelerated filer,” as defined in Rule 12b-2 promulgated under the Exchange Act, or (iv) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the previous three-years.
Critical Accounting and Estimates
The discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). The preparation of these consolidated financial statements requires us to make estimates, judgments and assumptions that affect the reported amounts of assets, liabilities, revenues, and expenses and the related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate these estimates, and assumptions, including those related to revenue, inventories and income taxes, among others. Our estimates are derived from historical experience, current conditions and various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities as well as identifying and assessing the accounting treatment with respect to commitments and contingencies. Our actual results may materially differ from
116
these estimates. In addition, any change in these estimates or their underlying assumptions could have a material adverse effect on our operating results.
We believe that the accounting policies discussed below are critical to the understanding of our historical and future performance, and these accounting policies involve a significant degree of judgment and complexity. For further information, see the accompanying notes to our audited consolidated financial statements.
Revenue Recognition
Revenue is recognized when a customer obtains control of promised goods or services. The amount of revenue recognized reflects the consideration we expect to be entitled to receive in exchange for these goods or services.
We determine revenue recognition through the following steps:
1.Identification of the contract, or contracts, with a customer:
We consider the terms and conditions of the contract in identifying the contracts. We determine a contract with a customer to exist when the contract is approved, each party’s rights regarding the goods or services to be transferred can be identified, the payment terms for the goods or services can be identified, it has been determined the customer has the ability and intent to pay, and the contract has commercial substance. At contract inception, we evaluate whether two or more contracts should be combined and accounted for as a single contract and whether the combined or single contract includes more than one performance obligation. We apply judgment in determining the customer’s ability and intent to pay, which is based on a variety of factors, including the customer’s historical payment experience or, in the case of a new customer, credit and financial information pertaining to the customer.
2.Identification of the performance obligations in the contract:
Performance obligations promised in a contract are identified based on the goods or services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the goods or services either on its own or together with other resources that are readily available from third parties or from us, and are distinct in the context of the contract, whereby the transfer of the goods or services is separately identifiable from other promises in the contract. Our performance obligations consist of (i) product sales, (ii) maintenance contracts and (iii) other services including training.
3.Determination of the transaction price:
The transaction price is determined based on the consideration to which we expect to be entitled in exchange for transferring goods or services to the customer. Variable consideration is included in the transaction price if, in our judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. Our contracts do not contain a significant financing component.
4.Allocation of the transaction price to the performance obligations in the contract:
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative standalone selling price.
5.Recognition of revenue when, or as a performance obligation is satisfied:
For product sales and services, revenue is recognized at the time the related performance obligation is satisfied by transferring the control of the promised goods or services to a customer, in an amount that reflects the consideration that we expect to receive in exchange for those goods or services. Training and maintenance services are generally recognized upon invoicing in amounts that correspond directly with the value to the customer of the performance completed to date which primarily includes professional service arrangements entered on a time and materials basis.
Inventory
Inventory is stated at the lower of cost or net realizable value. Cost is determined using the weighted- average cost method. We periodically reviews the value of items in inventory and provides write-offs of inventory that is obsolete. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating net realizable value. Once inventory has been written down below cost, it is not subsequently written up.
117
Income Taxes
Deferred tax assets and liabilities are determined based on the differences between financial reporting and the tax basis of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets be reduced by a valuation allowance if it is more-likely-than-not that some or all of the deferred tax asset will not be realized. We annually evaluate the realizability of deferred tax assets by assessing the valuation allowance and by adjusting the amount of such allowance, if necessary. The factors used to assess the likelihood of realization include our forecast of future taxable income and available tax planning strategies that could be implemented to realize the net deferred tax assets.
We recognize the financial statement benefit of a tax position only after determining that the relevant tax authority would more-likely-than-not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the consolidated financial statements is the largest benefit that has a greater than 50% likelihood of being realized upon ultimate settlement with the relevant tax authority. In accordance with this accounting policy, we recognize accrued interest and penalties related to unrecognized tax benefits as a component of income tax benefit.
Recent Accounting Pronouncements
Recent Accounting Pronouncements Adopted
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. ASU 2020-06 reduces the number of accounting models for convertible instruments and allows more contracts to qualify for equity classification. The Company adopted this guidance effective January 1, 2024, and there was no material impact on the Company’s consolidated financial statements upon adoption.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires the Company to disclose interim and annual disclosures on significant segment expenses and other segment related items and is applicable to companies with a single reportable segment. The Company adopted the annual disclosure requirements effective for the fiscal year ended December 31, 2024 on a retrospective basis with the interim disclosure requirements becoming effective in the first quarter of 2025. The required disclosures were included under Note 16—Segment Information in the accompanying notes to the consolidated financial statements.
Recent Accounting Pronouncements Not Yet Adopted
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires disclosure of specific categories in the effective tax rate reconciliation and additional information for reconciling items that meet a quantitative threshold and further disaggregation of income taxes paid for individually significant jurisdictions. This guidance is effective on a prospective or retrospective basis for annual periods beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact of this guidance on the disclosures within its consolidated financial statements.
In November 2024, the FASB issued ASU 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses. The standard requires that public business entities disclose additional information about specific expense categories in the notes to financial statements for interim and annual reporting periods. The standard will become effective for the Company’s 2027 annual consolidated financial statements and interim condensed consolidated financial statements thereafter and may be applied prospectively to periods after the adoption date or retrospectively for all prior periods presented in the financial statements, with early adoption permitted. The Company is currently evaluating the impact of this guidance on the disclosures within its consolidated financial statements.
Item 7A: Quantitative and Qualitative Disclosures About Market Risk
Not required.
118
Item 8: Financial Statements and Supplementary Data
INDEX TO FINANCIAL STATEMENTS
QT IMAGING HOLDINGS, INC. CONSOLIDATED FINANCIAL STATEMENTS
| Page | |||||
Report of Independent Registered Public Accounting Firm (PCAOB ID: | |||||
Consolidated Balance Sheets as of December 31, 2024 and 2023 | |||||
Notes to Consolidated Financial Statements | |||||
F-1
Report of Independent Registered Public Accounting Firm
Board of Directors and Stockholders of
QT Imaging Holdings, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of QT Imaging Holdings, Inc. (a Delaware corporation) and its subsidiaries (the “Company”) as of December 31, 2024 and 2023, and the related consolidated statements of operations and comprehensive loss, stockholders’ deficit, and cash flows for each of the two years in the period ended December 31, 2024, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2024 and 2023, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2024, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ BPM LLP
We have served as the Company’s auditor since 2022.
March 28, 2025
F-2
QT IMAGING HOLDINGS, INC.
Consolidated Balance Sheets
As of December 31, 2024 and 2023
| December 31, 2024 | December 31, 2023 | ||||||||||
| ASSETS | |||||||||||
| Current assets: | |||||||||||
| Cash | $ | $ | |||||||||
| Restricted cash and cash equivalents | |||||||||||
| Accounts receivable | |||||||||||
| Inventory | |||||||||||
| Prepaid expenses and other current assets | |||||||||||
| Total current assets | |||||||||||
| Property and equipment, net | |||||||||||
| Intangible assets, net | |||||||||||
| Operating lease right-of-use assets, net | |||||||||||
| Other assets | |||||||||||
| Total assets | $ | $ | |||||||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | |||||||||||
| Current liabilities: | |||||||||||
| Accounts payable | $ | $ | |||||||||
| Accrued expenses and other current liabilities | |||||||||||
| Related party notes payable | |||||||||||
| Current maturities of long-term debt | |||||||||||
| Deferred revenue | |||||||||||
| Operating lease liabilities, current | |||||||||||
| Total current liabilities | |||||||||||
| Long-term debt | |||||||||||
| Related party notes payable | |||||||||||
| Operating lease liabilities | |||||||||||
| Warrant liability | |||||||||||
| Derivative liability | |||||||||||
| Earnout liability | |||||||||||
| Related party interest payable | |||||||||||
| Total liabilities | |||||||||||
| Contingencies (Note 10) | |||||||||||
| Stockholders’ deficit: | |||||||||||
Preferred stock, $ | |||||||||||
Common stock, $ | |||||||||||
| Additional paid-in capital (1) | |||||||||||
| Accumulated deficit | ( | ( | |||||||||
| Total stockholders’ deficit | ( | ( | |||||||||
| Total liabilities and stockholders’ deficit | $ | $ | |||||||||
(1)Amounts as of December 31, 2023 differ from those in prior year consolidated financial statements as they were retrospectively adjusted as a result of the accounting or the Business Combination (as defined in the Notes to Consolidated Financial Statements).
The accompanying notes are an integral part of these consolidated financial statements.
F-3
QT IMAGING HOLDINGS, INC.
Consolidated Statements of Operations and Comprehensive Loss
For the years ended December 31, 2024 and 2023
| Year Ended December 31, | |||||||||||
| 2024 | 2023 | ||||||||||
| Revenue | $ | $ | |||||||||
| Cost of revenue | |||||||||||
| Gross profit (loss) | ( | ||||||||||
| Operating expenses: | |||||||||||
| Research and development | |||||||||||
| Selling, general and administrative | |||||||||||
| Total operating expenses | |||||||||||
| Loss from operations | ( | ( | |||||||||
| Other expense, net | ( | ( | |||||||||
| Change in fair value of warrant liability | |||||||||||
| Change in fair value of derivative liability | |||||||||||
| Change in fair value of earnout liability | |||||||||||
| Interest expense, net | ( | ( | |||||||||
| Loss before income tax expense (benefit) | ( | ( | |||||||||
| Income tax expense (benefit) | ( | ||||||||||
| Net loss and comprehensive loss attributable to QT Imaging Holdings, Inc. | ( | ( | |||||||||
| Less: deemed dividend related to the modification of equity classified warrants | ( | ||||||||||
| Net loss and comprehensive loss attributable to common stockholders | $ | ( | $ | ( | |||||||
| Net loss per share - basic and diluted (1) | $ | ( | $ | ( | |||||||
| Weighted-average number of common shares used in computing net loss per common share (1) | |||||||||||
(1)Amounts for the year ended December 31, 2023 and before that date differ from those in prior year consolidated financial statements as they were retrospectively adjusted as a result of the accounting or the Business Combination (as defined in the Notes to Consolidated Financial Statements).
The accompanying notes are an integral part of these consolidated financial statements.
F-4
QT IMAGING HOLDINGS, INC.
Consolidated Statements of Stockholders’ Deficit
For the years ended December 31, 2024 and 2023
| Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total | ||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||
| Balance, January 1, 2024 | $ | $ | $ | ( | $ | ( | |||||||||||||||||||||||
| Reverse recapitalization | ( | ( | — | ||||||||||||||||||||||||||
| As adjusted, beginning of period (1) | ( | ( | |||||||||||||||||||||||||||
| Merger recapitalization | ( | — | ( | ||||||||||||||||||||||||||
| Issuance of common stock pursuant to a subscription agreement | — | ||||||||||||||||||||||||||||
| Conversion of a note payable | — | ||||||||||||||||||||||||||||
| Conversion of a bridge loan | — | ||||||||||||||||||||||||||||
| Net exercise of warrants | ( | — | |||||||||||||||||||||||||||
| Issuance of common stock in connection with the Pre-Paid Advance | — | ||||||||||||||||||||||||||||
| Issuance of common stock in connection with the Cable Car Loan | — | ||||||||||||||||||||||||||||
| Issuance of common stock related to non-redemption extension agreements | — | ||||||||||||||||||||||||||||
| Issuance of common stock related to early investor consideration | — | ||||||||||||||||||||||||||||
| Issuance of common stock to settle transaction expenses | — | ||||||||||||||||||||||||||||
| Issuance of common stock in exchange for services | — | ||||||||||||||||||||||||||||
| Conversion of long term debt into shares of common stock | — | ||||||||||||||||||||||||||||
| Conversion of related party extension note into shares of common stock | — | ||||||||||||||||||||||||||||
| Issuance of common stock related to PIPE with related parties | — | ||||||||||||||||||||||||||||
| Issuance of warrants related to PIPE with related parties | — | — | — | ||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | ||||||||||||||||||||||||||
| Deemed dividend related to modification of equity classified warrants | — | — | ( | ||||||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Balance, December 31, 2024 | $ | $ | $ | ( | $ | ( | |||||||||||||||||||||||
(1)Amounts as of December 31, 2023 and before that date differ from those in prior year consolidated financial statements as they were retrospectively adjusted as a result of the accounting or the Business Combination (as defined in the Notes to Consolidated Financial Statements).
The accompanying notes are an integral part of these consolidated financial statements.
F-5
QT IMAGING HOLDINGS, INC.
Consolidated Statements of Stockholders’ Deficit
For the years ended December 31, 2024 and 2023
| Common Stock | Additional Paid-in Capital | Accumulated Deficit | Total | ||||||||||||||||||||||||||
| Shares | Amount | ||||||||||||||||||||||||||||
| Balance, January 1, 2023 | $ | $ | $ | ( | $ | ( | |||||||||||||||||||||||
| Reverse recapitalization | ( | ( | — | ||||||||||||||||||||||||||
| As adjusted, beginning of period (1) | ( | ( | |||||||||||||||||||||||||||
| Sale of common stock and warrants in private offering, net | — | ||||||||||||||||||||||||||||
| Issuance of common stock for the conversion of notes payable plus accrued interest | — | ||||||||||||||||||||||||||||
| Stock-based compensation | — | — | — | ||||||||||||||||||||||||||
| Fair value of warrants | — | — | — | ||||||||||||||||||||||||||
| Net loss | — | — | — | ( | ( | ||||||||||||||||||||||||
| Balance, December 31, 2023 | $ | $ | $ | ( | $ | ( | |||||||||||||||||||||||
(1)Amounts as of December 31, 2023 and before that date differ from those in prior year consolidated financial statements as they were retrospectively adjusted as a result of the accounting or the Business Combination (as defined in the Notes to Consolidated Financial Statements).
The accompanying notes are an integral part of these consolidated financial statements.
F-6
QT IMAGING HOLDINGS, INC.
Consolidated Statements of Cash Flows
For the years ended December 31, 2024 and 2023
| Year Ended December 31, | |||||||||||
| 2024 | 2023 | ||||||||||
| Cash flows from operating activities: | |||||||||||
| Net loss | $ | ( | $ | ( | |||||||
| Adjustment to reconcile net loss to net cash used in operating activities: | |||||||||||
| Depreciation and amortization | |||||||||||
| Stock-based compensation | |||||||||||
| Provision for credit losses | |||||||||||
| Fair value of common stock issued in exchange for services and in connection with non-redemption agreements | |||||||||||
| Induced conversion expense | |||||||||||
| Debt conversion loss | |||||||||||
| Loss on issuance of common stock in connection with a subscription agreement | |||||||||||
| Warrant modification expense | |||||||||||
| Loss on debt extinguishment | |||||||||||
| Non-cash interest | |||||||||||
| Non-cash operating lease expense | ( | ( | |||||||||
| Loss on disposal of assets | |||||||||||
| Change in fair value of warrant liability | ( | ||||||||||
| Change in fair value of derivative liability | ( | ||||||||||
| Change in fair value of earnout liability | ( | ||||||||||
| Changes in operating assets and liabilities: | |||||||||||
| Accounts receivable | ( | ( | |||||||||
| Inventory | |||||||||||
| Prepaid expenses and other current assets | ( | ( | |||||||||
| Other assets | |||||||||||
| Accounts payable | ( | ||||||||||
| Accrued expenses and other current liabilities | ( | ||||||||||
| Deferred revenue | ( | ||||||||||
| Other liabilities | ( | ||||||||||
| Net cash used in operating activities | ( | ( | |||||||||
| Cash flows from investing activities: | |||||||||||
| Purchases of property and equipment | ( | ( | |||||||||
| Net cash used in investing activities | ( | ( | |||||||||
| Cash flows from financing activities: | |||||||||||
| Proceeds from sale of common stock and warrants, net of issuance costs | |||||||||||
| Proceeds from issuance common stock pursuant to subscription agreement | |||||||||||
| Proceeds from long-term debt, net of issuance costs | |||||||||||
| Repayment of long-term debt | ( | ( | |||||||||
| Repayment of bridge loans | ( | ||||||||||
| Proceeds from related party payable | |||||||||||
| Proceeds from the Merger, net of transaction costs | |||||||||||
| Cash paid for debt issuance costs | ( | ||||||||||
| Cash paid to lender for debt modification | ( | ||||||||||
| Net cash provided by financing activities | |||||||||||
| Net increase (decrease) in cash and restricted cash and cash equivalents | ( | ||||||||||
| Cash and restricted cash and cash equivalents, beginning of year | |||||||||||
| Cash and restricted cash and cash equivalents, end of year | $ | $ | |||||||||
| Supplemental disclosure of cash flow information: | |||||||||||
| Cash paid for interest | $ | $ | |||||||||
| Supplemental disclosures of noncash investing and financing activities: | |||||||||||
| Fair value of embedded derivatives upon issuance of convertible debt | $ | $ | |||||||||
| Fair value of earnout liability at issuance | |||||||||||
| Fair value of common stock issued with convertible debt | |||||||||||
| Conversion of Extension Note into common stock | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-7
QT IMAGING HOLDINGS, INC.
Consolidated Statements of Cash Flows
For the years ended December 31, 2024 and 2023
| Conversion of Yorkville Note into common stock | |||||||||||
| Extinguishment of accrued expenses in exchange for common stock | |||||||||||
| Transfer of equipment to inventory | |||||||||||
| Transfer of inventory to property and equipment | |||||||||||
| Debt discount included in accrued expenses | |||||||||||
| Debt discount included in accounts payable | |||||||||||
| Conversion of long-term debt into common stock | |||||||||||
| Deemed dividend | |||||||||||
| Purchase of property and equipment included in accounts payable | |||||||||||
| Related party convertible notes payable including accrued interest exchanged for common stock | |||||||||||
| Transfer of accrued interest to current maturities of long-term debt | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
F-8
1.The Company and Summary of Significant Accounting Policies
Nature of Operations
QT Imaging Holdings, Inc. and its subsidiaries (the “Company”), formerly known as GigCapital5, Inc. (“GigCapital5”), is incorporated in Delaware with headquarters in Novato, California. The Company is a medical device company engaged in research, development, and commercialization of innovative body imaging systems using low frequency sound waves. The Company strives to improve global health outcomes. Its strategy is predicated upon the fact that medical imaging is critical to the detection, diagnosis, and treatment of disease and that it should be safe, affordable, accessible, and centered on the patient’s experience. The Company’s initial product is a breast imaging system.
On March 4, 2024 (the “Closing Date” or “Merger Date”), QT Imaging, Inc. (“QT Imaging”), GigCapital5, and QTI Merger Sub, Inc. (“QTI Merger Sub”) pursuant to the terms of the Business Combination Agreement (the “Business Combination Agreement”) dated December 8, 2022, completed the business combination of QT Imaging and GigCapital5 which was effected by the merger of QTI Merger Sub with and into QT Imaging, with QT Imaging surviving the Merger as a wholly owned subsidiary of GigCapital5 (the “Merger,” and, together with the other transaction contemplated by the Business Combination Agreement, the “Business Combination”). Upon completion of the merger on March 4, 2024, GigCapital5 changed its name to QT Imaging Holdings, Inc. and effectively assumed all of QT Imaging’s material operations. Refer to Note 2 - Business Combination for more information regarding the Merger.
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). In the opinion of management, the consolidated financial statements contain all adjustments necessary for a fair presentation of the Company’s financial position as of the date reported.
The share and per share amounts, prior to the Merger, have been retrospectively restated as shares reflecting conversion at the exchange ratio of approximately 0.3427 established in the Business Combination Agreement.
Principles of Consolidation
The consolidated financial statements include the accounts of QT Imaging Holdings, Inc. and its wholly-owned subsidiaries, QT Imaging and QT Ultrasound Labs, Inc. (“QT Labs”). All intercompany balances and transactions are eliminated in consolidation.
Liquidity
The Company has incurred net operating losses and negative cash flows from operations since its inception and had an accumulated deficit of $31,940,527 as of December 31, 2024. During the year ended December 31, 2024, the Company incurred a net loss of $8,984,880 and used $10,033,477 of cash in operating activities, which includes repayment of net liabilities assumed from the Business Combination. The Company expects to continue to incur losses, and its ability to achieve and sustain profitability will depend on the achievement of sufficient revenues to support the Company’s cost structure. The Company may never achieve profitability and, unless and until it does, the Company will need to continue to raise additional capital.
In connection with the Business Combination, the Company entered into various agreements to obtain financing through the issuance of debt and through stock subscription agreements. On March 4, 2024, the Company received the Pre-Paid Advance (as defined in Note 2), net of issuance costs, of $9,025,000 from YA II PN, LTD (“Yorkville”) pursuant to the Standby Equity Purchase Agreement (the “SEPA”) and issued Yorkville a promissory note (the “Yorkville Note”) in the amount of $10.0 million for such Pre-Paid Advance, $500,000 of cash proceeds from an investor related to a stock subscription agreement, and $1,500,000 in cash proceeds through a note payable from
F-9
Funicular Funds, LP. See Note 8. Long-Term Debt. The SEPA provides the Company with access to an additional $40 million of potential capital through the issuance of common stock to Yorkville. During the time the Company has a balance under the Yorkville Note, additional advances under the SEPA can be received with written consent of Yorkville or upon a Trigger Event (as defined in Note 8) which, following the effectiveness of the registration statement on Form S-1 that the Company filed to register the shares to be issued pursuant to the SEPA, occurs when the daily volume-weighted average price (“VWAP”) is less than the Floor Price (as such term is defined in the Yorkville Note) for five consecutive trading days, which prior to October 31, 2024, was $0.8768 per share. As previously disclosed in a Current Report on Form 8-K with the SEC on September 13, 2024, a Trigger Event occurred on September 11, 2024, following which on September 13, 2024, the Company made a payment to Yorkville on the Yorkville Note of $1,521,581 which included $1,145,407 as repayment of principal. Additionally, and as previously disclosed in a Current Report on Form 8-K with the SEC on September 30, 2024, the Company and Yorkville executed an amendment on September 26, 2024 to extend the maturity date of the Yorkville Note from June 4, 2025 to December 15, 2025 and decreased the monthly principal payment obligations of $500,000 related to the Trigger Event beginning on January 15, 2025 (see Note 8 for more detail). Subsequently, on October 31, 2024, the Company and Yorkville executed a second amendment to extend the maturity date of the Yorkville Note to March 31, 2026 and reduced the Floor Price to $0.50 per share. On November 12, 2024, the Company executed a securities purchase agreement with related parties for the issuance of shares of common stock plus warrants for the purchase of common stock as a Private Investment in Public Equity (the “PIPE”) with an aggregate purchase price of $2.56 million, the closing of which occured on November 22, 2024. On December 11, 2024, the Company and NXC Imaging (“NXC”) entered into the Amended Distribution Agreement (which was further amended on March 28, 2025), which provides the Company with minimum order quantities (“MOQs”) amounting to cash inflows of $18.0 million in 2025 and $27.0 million in 2026. On February 26, 2025, the Company entered into a credit agreement (the “Credit Agreement”) that provides a senior secured term loan (the “Lynrock Lake Term Loan”) with Lynrock Lake Master Fund LP (“Lynrock Lake”) for a term loan in the aggregate principal amount of $10.1 million and repaid the secured Cable Car Note, as defined in Note 8. Long-Term Debt, and fully settled its obligations under the Yorkville Note and terminated the Yorkville SEPA by paying $3.0 million in cash and issuing a 5-year warrant to purchase 15 million shares of common stock. Net of these payments, the Company had $5.4 million of net proceeds for working capital purposes. Management believes that the additional cash received for the Lynrock Lake Term Loan and additional revenue from MOQs per the Amended Distribution Agreement will be sufficient to fund the Company’s current operating plan for at least the next 12 months.
The Company’s future capital requirements will depend on many factors, including the Company’s growth rate, the timing and extent of its spending to support research and development activities, purchasing inventory to meet its growth plan, and the timing and cost to enhance commercialized existing products. In the event that additional financing is required from outside sources, the Company may not be able to raise it on terms acceptable to the Company, or at all. Any additional debt financing obtained by the Company in the future could also involve restrictive covenants relating to the Company’s capital-raising activities and other financial and operational matters, which may make it more difficult for the Company to obtain additional capital and to pursue business opportunities, including potential acquisitions. Additionally, if the Company raises additional funds through further issuances of equity, convertible debt securities or other securities convertible into equity, its existing stockholders could suffer significant dilution in their percentage ownership of the Company, and any new equity securities the Company issues could have rights, preferences and privileges senior to those of holders of the Company’s common stock. If the Company is unable to obtain adequate financing or financing on terms satisfactory to the Company when the Company requires it, the Company’s ability to continue to grow or support its business and to respond to business challenges could be significantly limited.
Reclassification
Certain reclassifications have been made to the prior year consolidated statement of operations and comprehensive loss to conform to the current year presentation. The reclassification had no impact on the previously reported consolidated balance sheet, statement of stockholders’ deficit or cash flows.
F-10
Use of Estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues, and expenses and the related disclosure of contingent assets and liabilities. The Company bases its estimates on historical experience and on various other assumptions that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates. In addition, any change in these estimates or their related assumptions could have an adverse effect on the Company’s operating results.
Business Risk and Concentration of Credit Risk and Supply Risk
Financial instruments that potentially subject the Company to significant concentrations of credit risk consist primarily of cash and accounts receivable. The majority of the Company’s cash is invested in U.S. dollar deposits with a reputable bank in the United States. Management believes that minimal credit risk exists with respect to the financial institution that holds the Company’s cash. At times, such cash may be in excess of insured limits established by the Federal Deposit Insurance Corporation.
The Company performs ongoing credit evaluations of its customers and generally does not require collateral for accounts receivable. Payment terms range from cash in advance to 30 days from delivery of products or services but may fluctuate depending on the terms of each specific contract.
Significant customers represent 10% or more of the Company’s total revenue or accounts receivable balance for the period ended as of each reporting date. For each significant customer, revenue as a percentage of total revenue and accounts receivable as a percentage of total accounts receivable are as follows:
| Revenue | |||||||||||||||||||||||
| Accounts Receivable | Year Ended December 31, | ||||||||||||||||||||||
| December 31, 2024 | December 31, 2023 | 2024 | 2023 | ||||||||||||||||||||
| Customers: | |||||||||||||||||||||||
| Customer A | % | * | % | * | |||||||||||||||||||
| Customer B | * | * | % | * | |||||||||||||||||||
| Customer C | % | * | * | * | |||||||||||||||||||
| Customer D, related party | * | * | * | % | |||||||||||||||||||
| Customer E | * | * | * | % | |||||||||||||||||||
| Customer F | * | % | * | * | |||||||||||||||||||
| Customer G | * | * | * | % | |||||||||||||||||||
| Customer H | * | * | * | % | |||||||||||||||||||
| Customer I | % | * | * | * | |||||||||||||||||||
| % | % | % | % | ||||||||||||||||||||
*Total less than 10% for the period.
There are inherent risks whenever a large percentage of total revenue is concentrated in a limited number of customers. Should a significant customer which is a party to a contract with the Company under which the Company derives revenue terminate or fail to renew its contracts with the Company, in whole or in part, for any reason, or experience significant financial or operating difficulties, it could have a material adverse effect on the Company’s financial condition and results of operations. In general, a customer that makes up a significant portion of revenues in one period, may not make up a significant portion in subsequent periods. However, as the Company has entered into a Distribution Agreement with NXC on June 18, 2024, and as amended on December 11, 2024, which was further amended on March 28, 2025, by which the Company appointed NXC as the exclusive reseller to market, advertise, and resell certain equipment in the U.S. and U.S. territories, the Company expects that NXC will make up a significant
F-11
portion of revenues in each period in which such Distribution Agreement is in effect. Customer A in the concentration table above is NXC, which resold the Company’s scanner to eight clinics during the year ended December 31, 2024.
Certain components and services used to manufacture and develop the Company’s products are presently available from only one or a limited number of suppliers or vendors. The Company’s QT Breast Scanner has more than six hundred components, of which less than five components have such dependencies on limited suppliers or vendors. The loss of any of these suppliers or vendors would potentially require a significant level of hardware and/or software development efforts to incorporate the products or services into the Company’s product.
Cash and Cash Equivalents
The Company considers all short-term investments with a maturity of three months or less when purchased to be cash equivalents. The Company had restricted cash equivalents of $20,000
Restricted Cash
Restricted cash is comprised of cash held in an account subject to a collateral agreement to be used for the Company’s corporate credit card program.
Accounts Receivable
Inventory
Inventory is stated at the lower of cost or net realizable value. Cost is determined using the weighted-average cost method. The Company periodically reviews the value of items in inventory and provides write-offs of inventory that is obsolete. Appropriate consideration is given to obsolescence, excessive levels, deterioration, and other factors in evaluating net realizable value. Once inventory has been written down below cost, it is not subsequently written up.
Property and Equipment, Net
Property and equipment, net are recorded at cost, less accumulated depreciation. Expenditures for major additions and improvements are capitalized and minor replacements, maintenance, and repairs are charged to current operations as incurred. When property and equipment are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is included in the results of operations for the respective period. Depreciation is provided over the estimated useful lives of the related assets using the straight-line method. Leasehold improvements are amortized over the lesser of the term of the related lease or the estimated useful lives of the assets.
Leases
The Company primarily enters into leases for office space that are classified as operating leases. The Company determines if an arrangement is or contains a lease at inception. The Company accounts for leases by recording right-of-use (“ROU”) assets and lease liabilities on the consolidated balance sheets in the captions operating lease right-of-use assets, net and operating lease liabilities, respectively. The lease term includes the non-cancelable period of the lease plus any additional periods covered by an option to extend that the Company is reasonably certain to exercise. The Company’s leases do not include substantial variable payments based on an index or rates. The Company’s lease agreements do not contain any significant residual value guarantees or material restrictive covenants.
F-12
The Company’s leases do not provide a readily determinable implicit discount rate. The Company’s incremental borrowing rate is estimated to approximate the interest rate on a collateralized basis with similar terms and payments, and in similar economic environments. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. The lease payments related to the next 12 months are included in operating lease liabilities, current on the consolidated balance sheets. The Company recognizes a single lease cost on a straight-line basis over the term of the lease, and the Company classifies all cash payments within operating activities in the consolidated statements of cash flows.
The Company did not
Intangible Assets, Net
The Company’s intangible assets are comprised of patents with a useful life of 12 years. Patents are amortized on a straight-line basis over their useful life.
Long-Lived Assets
Fair Value Measurements
The Company applies the requirements of the fair value measurements framework, which establishes a hierarchy for measuring fair value and requires enhanced disclosures about fair value measurements. The fair value measurement guidance clarifies that fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The fair value measurement guidance also requires disclosure about how fair value is determined for assets and liabilities and establishes a hierarchy in which these assets and liabilities must be grouped based on significant levels of inputs.
Debt and Debt Issuance Costs
The Company evaluates its financial instruments to determine if they are freestanding financial instruments. The Company also evaluates its convertible debt for embedded derivatives. Embedded provisions (like conversion options) are assessed to determine if they qualify as embedded derivatives that require separate accounting.
Debt issuance costs are recorded as a reduction to the carrying amount of the debt and are amortized to interest expense using the effective interest method. Debt is classified as short-term or long-term based on the term of the note.
Revenue Recognition
Revenue is recognized when a customer obtains control of promised goods or services. The amount of revenue recognized reflects the consideration the Company expects to be entitled to receive in exchange for these goods or services.
F-13
The Company determines revenue recognition through the following steps:
1)Identification of the contract, or contracts, with a customer
The Company considers the terms and conditions of the contract in identifying the contracts. The Company determines a contract with a customer to exist when the contract is approved, each party’s rights regarding the goods or services to be transferred can be identified, the payment terms for the goods or services can be identified, it has been determined the customer has the ability and intent to pay, and the contract has commercial substance. At contract inception, the Company will evaluate whether two or more contracts should be combined and accounted for as a single contract and whether the combined or single contract includes more than one performance obligation. The Company applies judgment in determining the customer’s ability and intent to pay, which is based on a variety of factors, including the customer’s historical payment experience or, in the case of a new customer, credit and financial information pertaining to the customer.
2)Identification of the performance obligations in the contract
Performance obligations promised in a contract are identified based on the goods or services that will be transferred to the customer that are both capable of being distinct, whereby the customer can benefit from the goods or services either on its own or together with other resources that are readily available from third parties or from the Company, and are distinct in the context of the contract, whereby the transfer of the goods or services is separately identifiable from other promises in the contract. The Company’s performance obligations consist of (i) product sales, (ii) maintenance contracts and (iii) other services including training.
3)Determination of the transaction price
The transaction price is determined based on the consideration to which the Company expects to be entitled in exchange for transferring goods or services to the customer. Variable consideration is included in the transaction price if, in the Company’s judgment, it is probable that a significant future reversal of cumulative revenue under the contract will not occur. The Company’s contracts do not contain a significant financing component.
4)Allocation of the transaction price to the performance obligations in the contract
If the contract contains a single performance obligation, the entire transaction price is allocated to the single performance obligation. Contracts that contain multiple performance obligations require an allocation of the transaction price to each performance obligation based on a relative standalone selling price.
5)Recognition of revenue when, or as a performance obligation is satisfied
For product sales and services, revenue is recognized at the time the related performance obligation is satisfied by transferring the control of the promised goods or services to a customer, in an amount that reflects the consideration that the Company expects to receive in exchange for those goods or services. Training and maintenance services are generally recognized upon invoicing in amounts that correspond directly with the value to the customer of the performance completed to date which primarily includes professional service arrangements entered on a time and materials basis.
Substantially all of the revenue recognized by the Company during the years ended December 31, 2024 and 2023 was recognized at a point in time.
Revenue recognized during the years ended December 31, 2024 and 2023 is disaggregated as follows:
| Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
| Product | $ | $ | ||||||||||||
| Service | ||||||||||||||
| $ | $ | |||||||||||||
F-14
Revenue recognized by geography during the years ended December 31, 2024 and 2023 is as follows:
| Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
| United States | $ | $ | ||||||||||||
| International | ||||||||||||||
| $ | $ | |||||||||||||
The Company had no 49,365 as of December 31, 2024. The Company had contract liabilities of $347,619 as of December 31, 2023. Revenue recognized during the year ended December 31, 2024 that was previously included in contract liabilities as of December 31, 2023 was $39,683 , while a $300,000 customer deposit previously deferred was refunded due to an order cancellation during the year ended December 31, 2024.
Shipping and Handling Costs
Shipping and handling activities are typically performed before the customer obtains control of the goods, and the related costs are therefore expensed as incurred. Shipping and handling costs are included in cost of revenue in the accompanying consolidated statements of operations and comprehensive loss. Shipping and handling costs incurred for inventory purchases are expensed in cost of revenue when sold.
Product Warranty
The Company’s products sold to customers are generally subject to warranties up to twelve months , which provides for the repair or replacement of products, at the Company’s option, that fail to perform with stated specifications. The Company estimates future warranty obligations related to those products. To date, product warranty claims have not been significant.
Research and Development Costs
Advertising
Grant Income
Periodically, the Company is awarded grants on a cost reimbursement basis. Costs are expensed when incurred and reimbursable on a monthly or quarterly basis with the offset booked as a contra-expense to the applicable functional area in the consolidated statements of operations and comprehensive loss.
Income Taxes
Deferred tax assets and liabilities are determined based on the differences between financial reporting and the tax basis of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Deferred tax assets may be reduced by a valuation allowance if it is more-likely-than-not that some or all of the deferred tax asset will not be realized. The Company annually evaluates the realizability of deferred tax assets by assessing the valuation allowance and by adjusting the amount of such allowance, if necessary. The factors used to assess the likelihood of realization include the Company’s forecast of
F-15
future taxable income and available tax planning strategies that could be implemented to realize the net deferred tax assets.
Stock-Based Compensation
Stock-based compensation cost is measured at the grant date based on the fair market value of the award. Stock-based compensation is recognized as expense on a ratable basis over the requisite service period of the award.
The Company values stock options using the Black-Scholes option pricing model. This model requires the use of highly subjective and complex assumptions which determine the fair value of stock-based awards, including the option’s expected term, stock price volatility and risk-free interest rates. Forfeitures are recorded as they occur.
Comprehensive Loss
Net Loss per Share
Reconciliation of net loss per share for the years ended December 31, 2024 and 2023 is as follows:
| Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
| Net loss and comprehensive loss attributable to QT Imaging Holdings, Inc. | $ | ( | $ | ( | ||||||||||
| Deemed dividend related to the modification of equity classified warrants | ( | |||||||||||||
| Net loss attributable to common stockholders | $ | ( | $ | ( | ||||||||||
| Weighted-average number of common shares used in computing net loss per common share (1) | ||||||||||||||
| Net loss per share - basic and diluted (1) | $ | ( | $ | ( | ||||||||||
F-16
The following securities were excluded from the calculation of net loss per share because the inclusion would be anti-dilutive as of December 31, 2024 and 2023:
| December 31, 2024 | December 31, 2023 | ||||||||||
| Common stock warrants (1) | |||||||||||
| Potential shares from Pre-Paid Advance | |||||||||||
| Merger consideration earnout shares | |||||||||||
| Potential shares from Cable Car Loan | |||||||||||
| Potential shares from convertible notes (1) | |||||||||||
| Contingently issuable shares to GigCapital5 stockholders (1) | |||||||||||
| Options outstanding (1) | |||||||||||
(1)Amounts as of December 31, 2023 and before that date differ from those in prior year consolidated financial statements as they were retrospectively adjusted as a result of the accounting or the Business Combination (as defined in the Notes to Consolidated Financial Statements).
Fair Value of Financial Instruments
The fair value of the Company’s financial instruments, including cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable, and accrued expenses and other current liabilities approximate their fair values because of the relatively short maturity of these instruments. The carrying value of the Company’s borrowings approximates fair value based on current rates offered to the Company for instruments with similar terms.
Recent Accounting Pronouncements Adopted
In August 2020, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity. ASU 2020-06 reduces the number of accounting models for convertible instruments and allows more contracts to qualify for equity classification. The Company adopted this guidance effective January 1, 2024, and there was no material impact on the Company’s consolidated financial statements upon adoption.
In November 2023, the FASB issued ASU 2023-07, Segment Reporting (Topic 280): Improvements to Reportable Segment Disclosures, which requires the Company to disclose interim and annual disclosures on significant segment expenses and other segment related items and is applicable to companies with a single reportable segment. The Company adopted the annual disclosure requirements effective for the fiscal year ended December 31, 2024 on a retrospective basis with the interim disclosure requirements becoming effective in the first quarter of 2025. The required disclosures were included in Note 16—Segment Information.
Recent Accounting Pronouncements
In December 2023, the FASB issued ASU 2023-09, Income Taxes (Topic 740): Improvements to Income Tax Disclosures, which requires disclosure of specific categories in the effective tax rate reconciliation and additional information for reconciling items that meet a quantitative threshold and further disaggregation of income taxes paid for individually significant jurisdictions. This guidance is effective on a prospective or retrospective basis for annual periods beginning after December 15, 2024, with early adoption permitted. The Company is currently evaluating the impact of this guidance on the disclosures within its consolidated financial statements.
In November 2024, the FASB issued ASU 2024-03, Income Statement—Reporting Comprehensive Income—Expense Disaggregation Disclosures (Subtopic 220-40): Disaggregation of Income Statement Expenses. The standard requires that public business entities disclose additional information about specific expense categories in the notes to financial statements for interim and annual reporting periods. The standard will become effective for the Company’s 2027
F-17
annual consolidated financial statements and interim condensed consolidated financial statements thereafter and may be applied prospectively to periods after the adoption date or retrospectively for all prior periods presented in the financial statements, with early adoption permitted. The Company is currently evaluating the impact of this guidance on the disclosures within its consolidated financial statements.
2.Business Combination
As described in Note 1, the Merger with GigCapital5 was consummated on March 4, 2024. On the Merger Date, QT Imaging, GigCapital5, and QT Merger Sub, consummated the closing of the transactions contemplated by the Business Combination Agreement, following the approval at an annual stockholder meeting of the stockholders of GigCapital5 held on February 20, 2024 (the “Stockholder Meeting”).
The Business Combination was accounted for as a reverse recapitalization. Under this method of accounting, GigCapital5 was treated as the acquired company for financial reporting purposes. Accordingly, for accounting purposes, the Business Combination was treated as the equivalent of QT Imaging issuing shares of the net assets of GigCapital5, accompanied by a recapitalization. The shares and net loss per common share prior to the Merger have been retroactively restated as shares reflecting the exchange ratio established in the Merger (approximately 0.3427 shares of the Company's common stock for each share of QT Imaging common stock). The net liabilities of GigCapital5 have been recognized at carrying value, with no goodwill or other intangible assets recorded.
QT Imaging has been determined to be the accounting acquirer based on evaluation of the following facts and circumstances:
•QT Imaging's stockholders have a majority of the voting power of the Company;
•The majority of QT Imaging's board of directors continued to serve as directors of the Company;
•The majority of QT Imaging's management continued to serve as management of the Company;
•QT Imaging comprises the ongoing operations of the Company; and
•QT Imaging is the larger entity based on historical business activity and the larger employee base.
The following summarizes the elements of the Merger to the consolidated statements of stockholders’ deficit and cash flows, including the transaction funding, sources, and uses of cash:
| Recapitalization | |||||
| Cash in GigCapital5 Trust Account, net of redemptions | $ | ||||
| Plus: cash in GigCapital5 operating bank account | |||||
| Less: Payments made pursuant to non-redemption agreements | ( | ||||
| Less: GigCapital5 transaction costs paid from Trust | ( | ||||
| Less: Repayment of GigCapital5 related party notes | ( | ||||
| Net cash proceeds from GigCapital5 | |||||
| Assumed net liabilities from GigCapital5 including the initial recognition of the earnout liability, excluding net cash proceeds | ( | ||||
| Net impact of the Merger on the consolidated statement of stockholders' deficit | $ | ( | |||
Merger Related Activities
On November 15, 2023, GigCapital5, QT Imaging and Yorkville, a Cayman Islands exempt limited partnership managed by Yorkville Advisors Global, LP, entered into the SEPA. Upon the closing of the Merger, the Company has the right, provided there is no balance outstanding under the Yorkville Note (as defined below) or, if there is a balance outstanding under a Yorkville Note, with Yorkville’s prior written consent, or upon the occurrence of certain trigger events, to issue and sell to Yorkville, and Yorkville shall purchase from the Company, up to $10,000,000 in aggregate gross purchase price (the “Commitment Amount”) of newly issued shares of the common stock (each such sale, an “Advance”) by delivering written notice to Yorkville (each, an “Advance Notice” and the date on which the Company is deemed to have delivered an Advance Notice, the “Advance Notice Date”). As consideration for a payment of
F-18
$10,000,000 (the “Pre-Paid Advance”) received on March 4, 2024, the Company issued the Yorkville Note, which was issued with a 6 % original issue discount. The Yorkville Note for the Pre-Paid Advance was originally due 15 months from the date of issuance, and interest accrues on the outstanding balance of the Yorkville Note at an annual rate equal to 6 %, subject to an increase to 18 % upon an event of default. The Yorkville Note is convertible by Yorkville into shares of the Company’s common stock. On March 4, 2024, immediately prior to, and substantially concurrently with, the closing of the Business Combination, QT Imaging issued to Yorkville that number of shares of the Company which converted in the aggregate into 1,000,000 shares of the Company's common stock (the “Company Shares”) upon the completion of the Merger. See Note 8.
In February 2024, GigCapital5 and QT Imaging entered into a Note Purchase Agreement (the “Cable Car Loan”) with Funicular Funds, LP (“Cable Car”), pursuant to which Cable Car agreed to advance $1,500,000 at the closing of the Business Combination, as was evidenced by a promissory note that may be convertible in certain circumstances into shares of the Company's common stock at a conversion price of $2.00 per share (the “Loan”), dated March 4, 2024, by and between QT Imaging and Cable Car. The Loan does not bear interest, and is due and payable 13 months after issuance, unless the time for payment is accelerated as a result of an event of default. On March 4, 2024, as full compensation to Cable Car for the Loan to QT Imaging in lieu of any simple or in-kind interest on the Loan, QT Imaging issued to Cable Car that number of shares of the Company which at the completion of the Business Combination would be converted in accordance with the terms of the Business Combination Agreement into 180,000 shares of the Company's common stock. See Note 8.
In February 2024, GigCapital5 and QT Imaging (together the “parties”) entered into a subscription agreement with William Blair & Co., L.L.C. (“William Blair”) for the purchase of shares of common stock of QT Imaging. Pursuant to the subscription agreement, QT Imaging issued to William Blair in satisfaction of certain fees owed to William Blair for its services to the parties, that number of shares of QT Imaging which at the completion of the Business Combination were converted in accordance with the terms of the Business Combination Agreement into 740,000 shares of the Company’s common stock. The issuance of these shares settled $2,410,000 of net assumed liabilities from the business combination with an additional transaction cost expense of $202,200 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
In February 2024, the parties agreed to amend one of the non-redemption agreements that were entered into in December 2023 (“December 2023 Non-Redemption Agreements”), pursuant to which, and in addition to the Company’s common stock issuable Mizuho Securities USA, LLC (“Mizuho”) under the December 2023 Non-Redemption Agreement, Mizuho received from QT Imaging, in exchange for $250,000 of services rendered by Mizuho, that number of QT Imaging’s common stock that converted in accordance with the terms of the Business Combination Agreement into 100,000 shares of the Company’s common stock. The issuance of these shares settled $250,000 of net assumed liabilities from the business combination with an additional transaction expense of $103,000 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
In February 2024, QT Imaging and GigCapital5 entered into two additional subscription agreements with each of Donnelley Financial Solutions, LLC (“DFIN”) and IB Capital LLC (“iBankers”), dated as of February 23, 2024 and February 22, 2024, respectively (together, the “Subscription Agreements”), for the purchase of shares of common stock of QT Imaging. Pursuant to the Subscription Agreements, QT Imaging issued to each of DFIN and iBankers in satisfaction of $500,000 and $600,000 of fees owed to DFIN and iBankers, respectively, for their services, that number of shares of QT Imaging which at the completion of the Business Combination were converted in accordance with the terms of the Business Combination Agreement into 200,000 and 240,000 respective shares of the Company’s common stock. The issuance of these shares settled $1,100,000 of net assumed liabilities from the business combination with an additional transaction expense of $453,200 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
In February 2024, QT Imaging and LionBay Ventures (“LionBay”) entered into a Settlement and Termination Agreement (“Termination Agreement”). Pursuant to the terms of the Termination Agreement, QT Imaging terminated its Service Agreement with LionBay dated May 18, 2021 and the First Amendment of the Service Agreement dated September 9, 2021 (collectively as “Service Agreement”). In exchange for the termination of the Service Agreement and the termination of options to purchase 17,000 shares of common stock with a strike price of $8.50 per option that
F-19
were issued as part of the Service Agreement, QT Imaging agreed to issue that number of shares that converted into 10,000 shares of the Company’s common stock. The issuance of these shares resulted in an additional transaction expense of $35,300 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
In February 2024, QT Imaging received $500,000 in exchange for that number of shares that converted into 200,000 shares of the Company's common stock in accordance with the terms of the subscription agreement and Business Combination Agreement. The issuance of these shares resulted in an additional transaction expense of $206,000 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
Pursuant to an amendment dated December 13, 2023, between QT Imaging and Exit Strategy Partners, LLC (“Advisor”), the Company agreed to pay for Advisor’s services in exchange for that number of shares that converted into 250,000 shares of the Company’s common stock and a total cash amount of $225,000 , of which $125,000 was paid on the closing of the Business Combination on March 4, 2024 and the remaining $100,000 is due on the first anniversary of the closing of the Business Combination, which is recorded in accrued expenses and other current liabilities within the consolidated balance sheet as of December 31, 2024. The total cash consideration and issuance of shares related to this amendment resulted in a transaction expense of $1,107,500 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
On March 4, 2024, as consideration for the December 2023 Non-Redemption with certain GigCapital5 stockholders (“Non-Redeeming Stockholders”), QT Imaging issued that number of shares that converted into 427,477 shares of the Company’s common stock to the Non-Redeeming Stockholders. The issuance of these shares resulted in a transaction expense of $1,508,994 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
On March 4, 2024, the Company issued to subscribers to the Stock Subscription Agreements entered into in November 2023 equal to that number of shares that resulted in such parties as stockholders of QT Imaging receiving pursuant to the Business Combination Agreement 150,000 shares of the Company's common stock. The issuance of these shares resulted in a transaction expense of $529,500 recorded as selling, general and administrative expense within the consolidated statement of operations and comprehensive loss during the year ended December 31, 2024.
Merger Earnout Consideration Shares
Pursuant to the Second Amendment to Business Combination Agreement dated September 21, 2023, the Company is obliged to issue a maximum of 9,000,000 shares of Company's common stock (the “Merger Consideration Earnout Shares”) if certain triggering events and conditions are achieved during 2024, 2025, and 2026.
2024 Earnout Shares
Promptly following the date on which Company files its quarterly report on Form 10-Q with respect to its fiscal quarter ended September 30, 2024 with the SEC, an aggregate of 2,500,000 Merger Consideration Earnout Shares (the “2024 Earnout Shares”) will be issued to QT Imaging’s former stockholders if, and only if, on or prior to such filing date, the Company has obtained a formal Food and Drug Administration (“FDA”) clearance for breast cancer screening with respect to its breast scanning systems, which remains in full force and effect as of such filing date; provided, that the 2024 Earnout Shares shall increase by 500,000 (to an aggregate of 3,000,000 ) Merger Consideration Earnout Shares if, in addition, during the fifteen months ended September 30, 2024, the Company either (A) makes at least eight bona fide placements of its breast scanning systems globally or (B) has revenue of at least $4,400,000 as set forth in the condensed consolidated financial statements included in the periodic reports filed by the Company with the SEC with respect to such fifteen month period. These conditions were not met and therefore no shares were issued for the 2024 Earnout Shares during the year ended December 31, 2024.
F-20
2025 Earnout Shares
Promptly following the date on which the Company files its quarterly report on Form 10-Q with respect to its fiscal quarter ended September 30, 2025 with the SEC, an aggregate of 2,500,000 Merger Consideration Earnout Shares (the “2025 Earnout Shares”) will be issued to QT Imaging’s former stockholders if, and only if, during the twelve months ended September 30, 2025, (A) the Company achieves annual revenue of at least $17,100,000 as set forth in the condensed consolidated financial statements included in the periodic reports filed by the Company with the SEC with respect to such twelve month period, and (B) the Company makes at least four placements of its breast scanning systems in the United States; provided, that the 2025 Earnout Shares shall increase by 500,000 (to an aggregate of 3,000,000 ) Merger Consideration Earnout Shares if at least one of the following milestones is achieved: (x) on or prior to such filing date, the Company has obtained a formal FDA clearance for a new indication for use of its breast scanning systems (other than any indication obtained prior to the beginning of the twelve months ended September 30, 2025), which remains in full force and effect as of such filing date; or (y) the Company achieves clinical-quality patient images with the Company’s open angle scanner no later than the filing date of the quarterly report on Form 10-Q for the third quarter of 2025.
2026 Earnout Shares
Promptly following the date on which the Company files its quarterly report on Form 10-Q with respect to its fiscal quarter ended September 30, 2026 with the SEC, an aggregate of 2,500,000 Merger Consideration Earnout Shares (the “2026 Earnout Shares”) will be issued to QT Imaging’s former stockholders if, and only if, during the twelve months ended September 30, 2026, (A) the Company has revenue of at least $30,000,000 as set forth in the condensed consolidated financial statements included in the periodic reports filed by the Company with the SEC with respect to such twelve month period, or (B) the VWAP of shares of common stock equals or exceeds $15.00 per share for (20 ) of any (30 ) consecutive trading days on the Nasdaq exchange; provided, that the 2026 Earnout Shares shall increase by 500,000 (to an aggregate of 3,000,000 ) Merger Consideration Earnout Shares if at least one of the following milestones is achieved on or prior to such filing date: (x) the Company has obtained a formal FDA clearance of its open angle scanner, which remains in full force and effect as of such filing date; or (y) the Company receives net positive results in bona fide clinical trials, conducted in accordance with generally accepted industry standards, for its open angle scanner, as reported no later than the filing date of the quarterly report on Form 10-Q for the third quarter of 2026.
3.Fair Value Measurements
The fair value of the Company’s financial assets and liabilities reflects management’s estimate of amounts that the Company would have received in connection with the sale of the assets or paid in connection with the transfer of the liabilities in an orderly transaction between market participants at the measurement date. In connection with measuring the fair value of its assets and liabilities, the Company seeks to maximize the use of observable inputs (market data obtained from independent sources) and to minimize the use of unobservable inputs (internal assumptions about how market participants would price assets and liabilities). The following fair value hierarchy is used to classify assets and liabilities based on the observable inputs and unobservable inputs used in order to value the assets and liabilities:
Level 1: Quoted prices in active markets for identical assets or liabilities. An active market for an asset or liability is a market in which transactions for the asset or liability occur with sufficient frequency and volume to provide pricing information on an ongoing basis.
Level 2: Observable inputs other than Level 1 inputs. Examples of Level 2 inputs include quoted prices in active markets for similar assets or liabilities and quoted prices for identical assets or liabilities in markets that are not active.
Level 3: Unobservable inputs which are supported by little or no market activity and which are significant to the fair value of the assets or liabilities.
F-21
The following table presents information about the Company’s assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2024 and 2023, and indicates the fair value hierarchy of the valuation inputs the Company utilized to determine such fair value:
| Description: | Level | December 31, 2024 | December 31, 2023 | ||||||||||||||
| Assets: | |||||||||||||||||
| Certificate of deposit | 2 | $ | $ | ||||||||||||||
| Liabilities: | |||||||||||||||||
| Warrant liability | 2 | $ | $ | ||||||||||||||
| Earnout liability | 3 | $ | $ | ||||||||||||||
| Derivative liability | 3 | $ | $ | ||||||||||||||
Warrant Liability
The Company has determined that the warrants that were a constituent part of (i) the private placement units that were issued in a private placement sale by GigCapital5 prior to the Merger (“Private Placement Warrants”) and (ii) the private placement units that were issued upon conversion of working capital notes issued by GigCapital5 prior to the Merger, which conversion occurred concurrent with the Merger (“Working Capital Note Warrants”) are subject to treatment as a liability, as the transfer of the warrants to anyone other than the purchasers or their permitted transferees would result in these warrants having substantially the same terms as the warrants included in the public units that were issued by GigCapital5 prior to the Merger (“Public Warrants”). The Company determined that the fair value of each Private Placement Warrant and the Working Capital Note Warrants approximates the fair value of a Public Warrant. Accordingly, the Private Placement Warrants and Working Capital Note Warrants are valued upon observable data and have been classified as Level 2 financial instruments. As of December 31, 2024, a total of 889,364 Private Placement Warrants and Working Capital Note Warrants were outstanding at an approximate fair value of $0.025 per warrant. See Note 11.
The activity for the fair value of the warrant liability during the year ended December 31, 2024 was as follows:
| Warrant Liability | |||||
| Beginning balance, January 1, 2024 | $ | ||||
| Net liabilities assumed from GigCapital5 | |||||
| Increase due to warrant modification | |||||
| Change in fair value | ( | ||||
| Ending balance, December 31, 2024 | $ | ||||
The effect of the modification of the Private Placement Warrants and the Working Capital Note Warrants as further described in Note 11 was included within other expense, net in the consolidated statements of operations and comprehensive loss during the year ended December 31, 2024.
Earnout Liability
The fair value of the Merger Consideration Earnout shares was calculated using a Monte Carlo simulation. The simulation used as significant inputs the Company's management’s current assessment of placements of breast scanning systems in 2024 and 2025, likely expected values for revenues from 2024 through 2026, probabilities for regulatory approvals including FDA clearances, and probabilities of other triggering events related to the open angle scanner. The probabilities of the non-revenue triggers generally range from 0 to 25 percent. The revenue forecast for the respective measurement periods are generally in line with the revenue triggers as defined in the Business Combination Agreement, as amended. Additional significant inputs into the simulation include the volatility of Company's equity, assets, and revenue that was derived in a manner as would be common for such simulation, and
F-22
published industry operating profitability metrics. A weighted average cost of capital (“WACC”) was estimated based on a venture capital rates of return on debt and equity. This WACC was used as the discount rate applicable to revenue, after applying a delivering factor to convert it from being applicable to earnings before interest and tax (“EBIT”) to being applicable to revenue. This EBIT to revenue delivering factor was estimated using published industry operating profit and cost metrics.
The Monte Carlo simulation developed a distribution of projected revenues for 2024 through 2026 using a Geometric Brownian Motion framework based on a standard normal distribution of returns. The simulation also developed a distribution of potential daily common stock prices for 2026 using a Geometric Brownian Motion framework. The resulting fair value is based on the average of the number of shares that will be paid out for each triggering event over a statistically significant number of simulations.
Significant assumptions used in the valuation of the fair value of the earnout liability as of issuance on March 4, 2024 and as of December 31, 2024 were as follows:
| March 4, 2024 | December 31, 2024 | ||||||||||
| Fair value of common stock | $ | $ | |||||||||
| Volatility of revenue | % | % | |||||||||
| Discount rate applicable to revenue | % | % | |||||||||
| Risk-free rate | % | % | |||||||||
| Risk premium | % | % | |||||||||
| Cost of debt | % | % | |||||||||
| Credit risk spread | % | % | |||||||||
| Equity volatility | % | % | |||||||||
The activity for the fair value of the earnout liability for the year ended December 31, 2024 was as follows:
| Earnout Liability | |||||
| Beginning balance, January 1, 2024 | $ | ||||
| Fair value at issuance | |||||
| Change in fair value | ( | ||||
| Ending balance, December 31, 2024 | $ | ||||
Derivative Liability
In March 2024, the Company recorded a derivative liability related to the Pre-Paid Advance issued on March 4, 2024 pursuant to the SEPA, dated November 15, 2023, between QT Imaging and Yorkville (See Note 2 and Note 8). The Pre-Paid Advance contained the following derivative features (“Derivatives”) as defined in the SEPA that were recognized at fair value:
•Monthly Payment Premium: if, any time after the Issuance Date, and from time to time thereafter, a Trigger Event occurs, then the Company shall make monthly payments of Triggered Principal Amount, Payment Premium and accrued and unpaid interest.
•Monthly Payment Discount: if, any time after the Issuance Date, and from time to time thereafter, a Trigger Event occurs, then the Company shall make monthly payments of Triggered Principal Amount minus the lesser of (x) $1,500,000 and (y) such amount of fifty percent (50 %) of the Investor’s net sales proceeds of the Company Shares or fifty percent (50 %) of the value of the Company Shares on such date the cash payment is due.
•Variable Price Conversion Right: subject to certain limitations, at any time or times on or after the Issuance Date, the Yorkville shall be entitled to convert any portion of the outstanding and unpaid Conversion Amount into fully
F-23
paid and nonassessable common stock in accordance with Section (3)(b), at the Conversion Price of 95 % of the lowest VWAP of the Company’s common stock during the 5 consecutive Trading Days immediately preceding the Conversion Date or the date the Holder submits an Investor Notice pursuant to and as defined in the SEPA, as applicable, or other date of determination, but not lower than the Floor Price.
•Failure to Timely Convert: if within three (3 ) Trading Days after the Company’s receipt of an email copy of a Conversion Notice the Company shall fail to issue and deliver a certificate to the Yorkville or credit Yorkville’s balance account with DTC for the number of shares of common stock to which the Holder is entitled upon such Yorkville’s conversion of any Conversion Amount (a “Conversion Failure”), and if on or after such Trading Day the Yorkville purchases (in an open market transaction or otherwise) common stock to deliver in satisfaction of a sale by the Yorkville of common stock issuable upon such conversion that the Yorkville anticipated receiving from the Company (a “Buy-In”), then the Company shall, within three (3 ) Business Days after the Yorkville’s request and in the Yorkville’s discretion, either (i) pay cash to Yorkville in an amount equal to Yorkville’s total purchase price (including brokerage commissions and other out of pocket expenses, if any) for the common stock so purchased (the “Buy-In Price”), or (ii) promptly honor its obligation to deliver to the Yorkville a certificate or certificates representing such common stock and pay cash to the Yorkville in an amount equal to the excess (if any) of the Buy-In Price over the product of (A) such number of shares of common stock, times (B) the Closing Price on the Conversion Date.
•Corporate Events: in addition to and not in substitution for any other rights hereunder, prior to the consummation of any Fundamental Transaction pursuant to which holders of common stock are entitled to receive securities or other assets with respect to or in exchange for shares of common stock (a “Corporate Event”), the Company shall make appropriate provision to ensure that the Holder will thereafter have the right to receive upon a conversion of this Note, at the Holder’s option, (i) in addition to the common stock receivable upon such conversion, such securities or other assets to which the Holder would have been entitled with respect to such common stock had such common stock been held by the Holder upon the consummation of such Corporate Event (without taking into account any limitations or restrictions on the convertibility of this Note) or (ii) in lieu of the common stock otherwise receivable upon such conversion, such securities or other assets received by the holders of common stock in connection with the consummation of such Corporate Event in such amounts as the Holder would have been entitled to receive had this Note initially been issued with conversion rights for the form of such consideration (as opposed to common stock) at a conversion rate for such consideration commensurate with the Conversion Price. Provision made pursuant to the preceding sentence shall be in a form and substance satisfactory to the Required Holders.
The initial fair value of the above Derivatives was calculated using a Monte Carlo simulation. The simulation used significant inputs, including volatility of Company's equity that was derived based on a comparable peer group of publicly traded companies and the company’s stock price on the valuation date.
The total value of the derivatives reflected the combined value of the monthly payment premium, reduction to that premium by the payment discount, and the value of the conversion right. The values of the failure to timely convert and corporate event features were deemed to be de minimis.
Significant assumptions used in the valuation of the fair value of the derivative liability as of issuance on March 4, 2024 and as of December 31, 2024 were as follows:
| March 4, 2024 | December 31, 2024 | ||||||||||
| Fair value of common stock | $ | $ | |||||||||
| Term in years | |||||||||||
| Volatility | % | % | |||||||||
| Risk-free rate | % | % | |||||||||
| Debt discount | % | % | |||||||||
F-24
The activity for the fair value of the derivative liability during the year ended December 31, 2024 was as follows:
| Derivative Liability | |||||
| Beginning balance, January 1, 2024 | $ | ||||
| Fair value at issuance | |||||
| Change in fair value | ( | ||||
| Ending balance, December 31, 2024 | $ | ||||
4.Inventory
Inventory consisted of the following as of December 31, 2024 and 2023:
| December 31, 2024 | December 31, 2023 | ||||||||||
| Raw materials | $ | $ | |||||||||
| Work in process | |||||||||||
| Finished Goods | |||||||||||
| Total | $ | $ | |||||||||
5.Property and Equipment, Net
Property and equipment, net consisted of the following as of December 31, 2024 and 2023:
| Useful Life | December 31, 2024 | December 31, 2023 | |||||||||||||||
| Scanners | $ | $ | |||||||||||||||
| Computer and lab equipment | |||||||||||||||||
| Leasehold improvements | Various | ||||||||||||||||
| Software | |||||||||||||||||
| Furniture and fixtures | |||||||||||||||||
| Less: accumulated depreciation | ( | ( | |||||||||||||||
| $ | $ | ||||||||||||||||
6.Intangible Assets, Net
Intangible assets, net consisted of the following as of December 31, 2024:
| Useful Life | Gross Carrying Value | Accumulated Amortization | Net Carrying Value | Useful Life Remaining | |||||||||||||||||||||||||
| Patents | $ | $ | $ | ||||||||||||||||||||||||||
Intangible assets, net consisted of the following as of December 31, 2023:
F-25
| Useful Life | Gross Carrying Value | Accumulated Amortization | Net Carrying Value | Useful Life Remaining | |||||||||||||||||||||||||
| Patents | $ | $ | $ | ||||||||||||||||||||||||||
Amortization expense was $90,139 and $185,881 for each of the years ended December 31, 2024 and 2023.
7.Balance Sheet Details
Prepaid expenses and other current assets consisted of the following as of December 31, 2024 and 2023:
| December 31, 2024 | December 31, 2023 | ||||||||||
| Prepaid insurance | $ | $ | |||||||||
| Other | |||||||||||
| Total | $ | $ | |||||||||
Accrued expenses and other current liabilities consisted of the following as of December 31, 2024 and 2023:
| December 31, 2024 | December 31, 2023 | ||||||||||
| Accrued legal | $ | $ | |||||||||
| Accrued personnel costs | |||||||||||
| Accrued excise taxes | |||||||||||
| Accrued advisory fee | |||||||||||
| Other | |||||||||||
| Total | $ | $ | |||||||||
8.Long-Term Debt
Paycheck Protection Program Loan
On February 24, 2021 and May 5, 2020, the Company received loans (“PPP Loans”) from US Bank in the amounts of $1,158,265 (“Loan 2”) and $1,158,266 (“Loan 1”), respectively, to fund payroll, rent and utilities through the Paycheck Protection Program (“PPP”). Original loan terms were revised by the PPP Flexibility Act of 2020. Under the terms of the PPP, up to 100% of the loan and related interest was forgivable if the proceeds were used for covered expenses and certain other requirements related to wage rates were met. For Loan 1, the Company applied for forgiveness on June 7, 2021, and received forgiveness of $873,151 in principal and $9,823 in interest from the Small Business Administration (“SBA”) on June 14, 2021. For Loan 2, the Company applied for forgiveness on November 9, 2021, and received forgiveness of $930,246 in principal and $6,822 in interest on November 15, 2021.
The remaining balance of Loan 1 of $285,115 is payable in monthly installments of $6,400 , including interest at 1 %, beginning August 5, 2021, with the final payment due May 5, 2025. As of December 31, 2024, the total principal outstanding under Loan 1 was $31,920 , all of which was current. As of December 31, 2023, the total principal outstanding under Loan 1 was $107,979 , of which $76,058 was current and $31,921 was noncurrent.
The remaining balance of Loan 2 of $228,019 is payable in monthly installments of $4,605 , including interest at 1 %, beginning December 27, 2021, with the final payment due February 27, 2026. As of December 31, 2024, the total principal outstanding under Loan 2 was $64,061 , of which $54,864 was current and $9,197 was noncurrent. As of December 31, 2023, the total principal outstanding under Loan 2 was $118,369 , of which $54,308 was current and $64,061 was noncurrent.
Interest expense for Loan 1 and Loan 2 for the years ended December 31, 2024 and 2023 was $1,695 3,004
F-26
The SBA may undertake a review of a loan of any size during the six-year period following forgiveness or repayment of the loan. The review may include the loan forgiveness application, as well as whether the Company received the proper loan amount. The timing and outcome of any SBA review is not known.
Convertible Notes Payable
In June 2021, the Company entered into a convertible promissory note agreement (the “Note”) with USCG for advances of up to $10,000,000 . The Company could have made advances on the Note up to six months after the inception of the Note unless extensions for advances were mutually agreed between both parties. The Note bore interest at 12 % per annum on any amounts drawn with a maturity date of July 6, 2024. The Note was collateralized by all assets of the Company and was guaranteed by QT Labs. The terms of the Note include non-financial covenants and, as of March 4, 2024 when the Note converted, the Company was in compliance with those covenants. Through December 31, 2023, the Company issued warrants in connection with the note to purchase a total of 5,091 shares of common stock which 3,540 shares are exercisable at a price of $12.40 per share and 1,551 shares are exercisable at a price of $11.67 per share. The fair value of the warrants, along with financing fees, were recorded as debt issuance costs and presented in the consolidated balance sheets as a deduction from the carrying amount of the Note. On March 4, 2024, these warrants were terminated in accordance with the Business Combination Agreement.
The Note was convertible, at the Company’s option, before the Note matured upon the closing of a single transaction or a series of transactions with a minimum of $15,000,000 of cash proceeds raised in the aggregate. If elected, the conversion price is 90 % of the price per share in the qualified financing. Management assessed whether the embedded features in the Note should have been bifurcated from the debt host and concluded that none of the features required to be accounted for separately from the debt instrument.
In November 2023 and in connection with the Fourth Amendment and issuance of the senior secured convertible promissory note to US Capital as part of the Securities Purchase Agreement as described below (the “US Capital Note”), the outstanding loan balances of the Note of $2,495,000 with accrued interest of $635,854 were considered extinguished. In November 2023, the Company recorded $376,086 as a loss on extinguishment in other expense, net in the consolidated statements of operations and comprehensive loss, and includes a commission paid of $20,000 , remaining unamortized debt issuance costs on the Note of $32,828 and the fair value of warrants to purchase 16,320 shares of common stock of $156,505 .
As of December 31, 2024, there was no amount outstanding for the Note and US Capital Note. As of December 31, 2023, the total Note and US Capital Note balance was $3,294,659 net of unamortized debt issuance costs of $36,194 , and accrued interest of $50,037 . Interest expense, including amortization of debt issuance costs, for the years ended December 31, 2024 and 2023 was $88,692 and $340,758 , respectively.
On March 4, 2024, the Note principal and related accrued interest balance of $3,233,388 and the US Capital Note principal balance of $200,000 was converted into 359,266 and 100,000 shares of common stock, respectively. Additionally, warrants to purchase 16,320 shares of the Company's common stock were net settled into 5,594 shares of common stock.
Bridge Loan
In November 2023, the Company entered into a Securities Purchase Agreement and raised a private secured convertible bridge financing in the aggregate amount of $1,000,000 (“Bridge Loan”) from five investors (“Bridge Lenders”). Each Bridge Loan of $200,000 bore no interest but had a cash option value at the date of maturity of 120 %, or $240,000 , of the Bridge Loan at each Bridge Lender’s option. The maturity date was the closing date of the Business Combination as defined in Note 1. The Bridge Loan conversion price was at $2.00 per share on a post-business combination. On March 4, 2024, four of the five Bridge Loan holders elected the cash option and were paid an aggregate of $960,000 on the Merger Date. Interest expense related to the payment premium was $160,000 for the year ended December 31, 2024.
F-27
As of December 31, 2024, there was no amount outstanding for the Bridge Loan. As of December 31, 2023, the outstanding amount of the Bridge Loan, excluding the US Capital Note, was $774,337 , net of unamortized debt issuance costs of $25,663 . Interest expense from the amortization of debt issuance costs for the years ended December 31, 2024 and 2023 was $25,663 and $21,592 , respectively.
Yorkville Pre-Paid Advance
On March 4, 2024, the Company received the Pre-Paid Advance of $10,000,000 from Yorkville and issued Yorkville the Yorkville Note in the amount of $10,000,000 for such Pre-Paid Advance that was originally due 15 months from the date of issuance, and interest shall accrue on the outstanding balance of the Yorkville Note at an annual rate equal to 6 %, subject to an increase to 18 % upon an event of default as described in the Yorkville Note. The Yorkville Note is convertible by Yorkville into shares of the Company's common stock. As consideration for the Pre-Paid Advance, immediately prior to, and substantially concurrently with, the closing of the Business Combination, QT Imaging issued to Yorkville that number of shares of QT Imaging which converted in the aggregate into 1,000,000 shares of the Company's common stock upon the completion of the Business Combination. In accordance with Accounting Standards Codification (“ASC”) 470-20, the proceeds of $10,000,000 were recorded between the promissory note and common stock less debt origination costs of $975,000 , consisting of a $375,000 commitment fee for the SEPA and an original issue discount of 6 % for the Yorkville Note, on a relative fair value basis. Expenses related to a structuring fee was $20,000 for the year ended December 31, 2024, respectively, and was included in other expense, net in the consolidated statements of operations and comprehensive loss. As noted in Note 3, the Pre-Paid Advance contained Derivatives that were bifurcated and recorded a separate instrument. The initial value of the Derivatives of the $5,120,900 was recorded as a debt discount against the Pre-Paid Advance.
Under the terms of the original Yorkville Note, a “Trigger Event” shall occur if the daily VWAP is less than the Floor Price for five trading days during a period of seven consecutive trading days (a “Floor Price Trigger” and the last such day of such occurrence, a “Trigger Date”). If, at any time six months after the issuance of the Yorkville Note, a Trigger Event occurs, then the Company will be obligated to make monthly payments in an amount equal to the sum of (i) $1,500,000 of principal in the aggregate among all promissory notes issued to Yorkville (or the outstanding principal if less than such amount) (the “Triggered Principal Amount”), plus (ii) a payment premium of 5 % in respect of such Triggered Principal Amount, and (iii) accrued and unpaid interest hereunder as of each payment date beginning on the 5th trading day after the Trigger Date and continuing on the same day of each successive calendar month to Yorkville pursuant to the terms of the Yorkville Note. However, in the event that the Company shall be required to make such cash payments to Yorkville under the Yorkville Note as a result of the occurrence of a Trigger Event, the Company shall be entitled upon written notice to Yorkville, to direct that Yorkville (i) if Yorkville has sold the Company Shares that it received upon the completion of the Merger to apply, in accordance with the terms of the Yorkville Note, up to 50 % of Yorkville’s net sale proceeds of the Company Shares to satisfy, in part or in whole, the Triggered Principal Amount of such cash payments due to Yorkville or (ii) or if Yorkville has not sold the Company Shares, to apply up to 50 % of the value of the Company Shares on such date the cash payment is due based on the VWAP as quoted by Bloomberg LP of the Company Shares as an offset of the Triggered Principal Amount of the cash payments due to Yorkville. The obligation of the Company to make monthly prepayments due to the occurrence of a Floor Price Trigger shall cease (with respect to any payment that has not yet come due) if any time after the Trigger Date (a) the Company reduces the Floor Price to an amount that is at least 50 % of the daily VWAP of the common stock or (b) the daily VWAP is greater than 110 % of the Floor Price for a period of five consecutive trading days, unless a subsequent Trigger Event occurs. Furthermore, within one trading day of a Floor Price Trigger that remains after application of all amounts related to the Company Shares as described above, the Company shall reduce the Floor Price to an amount that is at least 50 % of the daily VWAP of the common stock, and provide Yorkville written confirmation of such reduction of the Floor Price or be obligated to make the above monthly cash payments.
Following the effectiveness of the registration statement on Form S-1 that the Company filed to register the shares to be issued pursuant to the SEPA, the Floor Price for Yorkville was $0.8768 per share. For the first five trading days commencing after six months after the issuance of the Yorkville Note, which ended on September 11, 2024, the daily VWAP of the common stock was less than the Floor Price, and as a result, September 11, 2024 constitutes a Trigger Date, and on that Trigger Date, a Trigger Event occurred due to a Floor Price Trigger. Accordingly, on September 13, 2024, the Company made the initial payment due to Yorkville as a result of the Trigger Event that occurred on September 11, 2024 in an amount totaling $1,521,581 , the calculation of which reflects a reduction to the Triggered
F-28
Principal Amount by 50 % of the net sale proceeds of the Company Shares by Yorkville following the closing of the Business Combination. The total payment of $1,521,581 comprised of $1,145,407 of principal, $318,904 of accrued interest, and $57,270 of 5 % early payment premium. The Company recognized the 5 % early payment premium as interest expense within the consolidated statements of operations and comprehensive loss during the year ended December 31, 2024.
On September 26, 2024, the Company and Yorkville entered into an Omnibus Amendment (the “Omnibus Amendment”), pursuant to which the Company and Yorkville agreed to amend certain terms of the Yorkville Note to reduce the Company’s obligations resulting from the occurrence of the Trigger Event. Pursuant to the Omnibus Amendment, the maturity date of the Yorkville Note was extended approximately six months from June 4, 2025 to December 15, 2025. Further, the Omnibus Amendment acknowledges the Company’s obligation to make monthly payments to Yorkville in the amount of the Trigger Principal Amount due to the occurrence of the Trigger Event and revised the Yorkville Note to provide that no further monthly payments will be owed during the period beginning on the date of the Omnibus Amendment and ending on January 15, 2025. In exchange for this relief, beginning on January 15, 2025, and continuing on the same day of each successive calendar month until and including November 15, 2025, whether or not a Trigger Event has occurred and is continuing as of such dates, the Company will make monthly payments in an amount equal to $500,000 plus the payment premium of 5 % plus accrued and unpaid interest under the Yorkville Note as of each such payment date. Such monthly payments will not be reduced or offset by any amount, including, but not limited to, any net sales proceeds of the Company Shares or any value of the Company Shares based on the VWAP as quoted by Bloomberg, LP. The Omnibus Amendment also provided that 100 % of the proceeds of the sale of the remaining 400,000 Company Shares held at the time of entry into the Omnibus Amendment by Yorkville shall be retained by Yorkville and shall not be used to offset or reduce any amounts owed under the Yorkville Note, or to otherwise benefit the Company in any way. The Omnibus Amendment also provides that in the event that the Company’s common stock is delisted from trading on the Nasdaq Stock Market, Yorkville consents to the occurrence of such delisting from the Nasdaq Stock Market, if it is to happen, and that it will not constitute an Event of Default as defined per the Omnibus Amendment, provided that (i) the Company uses its best efforts to have its common stock relisted on the Nasdaq Stock Market as soon as possible and (ii) the Company’s common stock is listed on the OTC Markets’ OTCQX market tier within 30 days in the event that a delisting from the Nasdaq Stock Market occurs. The Omnibus Amendment was accounted for as a troubled debt restructuring, resulting in a prospective adjustment to the effective interest rate in accordance with ASC 470-60.
On October 31, 2024, the Company and Yorkville executed the Second Omnibus Amendment to the Yorkville Note (the “Second Amendment”), pursuant to which the maturity date of the Yorkville Note was extended from December 15, 2025 to March 31, 2026. Further, the Second Amendment acknowledged the Company’s obligation to make monthly payments to Yorkville in the amount of the Trigger Principal Amount due to the occurrence of the Trigger Event and no further monthly payments will be owed during the period beginning on the date of the Second Amendment and ending on February 15, 2025. In exchange for this relief, beginning on February 15, 2025, and continuing on the same day of each successive calendar month until and including February 15, 2026, whether or not a Trigger Event has occurred and is continuing as of such dates, the Company agreed to make monthly payments in an amount equal to $500,000 plus the payment premium plus accrued and unpaid interest as of each such payment date. Such monthly payments under the Second Amendment were not to be reduced or offset by any amount, including, but not limited to, any net sales proceeds of the Company Shares or any value of the Company Shares based on the VWAP as quoted by Bloomberg, LP. Further, pursuant to the terms of the Second Amendment, the Company elected to reduce the Floor Price to $0.50 per share, effective as of the date of the Second Amendment. In addition, the Second Amendment provided that to the extent that Yorkville converts any portion of the Investor Note into shares of the common stock between the date of the Second Amendment and January 15, 2025, the first $500,000 of such conversions of the Yorkville Note shall reduce the principal balance of the Yorkville Note. For the avoidance of doubt and without implication that the opposite would otherwise be true, all other conversions of the Yorkville Note pursuant to the Second Amendment were to be applied as provided for in and consistent with the terms of the Yorkville Note. The Second Amendment also provided that in the event that the common stock is delisted from trading on the Nasdaq Stock Market, Yorkville consents to the occurrence of such delisting from the Nasdaq Stock Market, if it is to happen, and that it will not constitute an Event of Default as defined per the Omnibus Amendment, provided that (i) the Company uses its best efforts to have the common stock relisted on the Nasdaq Stock Market as soon as possible and (ii) the common stock is listed on the OTC Markets’ OTCQX or OTCQB market tiers within 30 days in the event that
F-29
a delisting from the Nasdaq Stock Market occurs. The Second Amendment was accounted for as a debt modification, resulting in a prospective adjustment to the effective interest rate.
On November 4, 2024, Yorkville converted $254,593 of outstanding principal into 384,059 shares of common stock with an applicable conversion price of $0.6629 per share. On December 6, 2024, Yorkville converted an additional $259,588 of outstanding principal under the Yorkville Note into 519,177 shares of common stock with an applicable conversion price of $0.50 per share.
As of December 31, 2024, the outstanding amount of the Yorkville Note was $3,532,591 , net of the unamortized debt discount of $4,807,820 and accrued interest of $155,203 . Interest expense, including amortization of debt issuance costs, for the year ended December 31, 2024 was $3,685,741 , of which $57,270 relates to the payment premium.
Cable Car Loan
In February 2024, GigCapital5 and QT Imaging entered into the Cable Car Loan with Cable Car, pursuant to which Cable Car agreed to advance $1,500,000 at the closing of the Business Combination, as was evidenced by the Loan, dated March 4, 2024, by and between QT Imaging and Cable Car. The Loan does not bear interest, and is due and payable 13 months after issuance, unless the time for payment is accelerated as a result of an event of default. As full compensation to Cable Car for the Loan to QT Imaging in lieu of any simple or in-kind interest on the Loan, QT Imaging issued to Cable Car that number of shares of QT Imaging which at the completion of the Business Combination would be converted in accordance with the terms of the Business Combination Agreement into 180,000 shares of the Company's common stock. In accordance with ASC 470-20, the proceeds of $1,500,000 were recorded between the promissory note and common stock less debt origination costs of $40,740 , consisting of legal fees, on a relative fair value basis.
As of December 31, 2024, the outstanding amount of the Cable Car Loan was $1,366,458 , net of unamortized issuance costs of $133,542 . Interest expense, including amortization of debt issuance costs, for the year ended December 31, 2024 was $353,530 .
Future principal payments on the long-term debt as of December 31, 2024 are as follows:
| Year ending December 31: | |||||
| 2025 | $ | ||||
| 2026 | |||||
| Total payments | |||||
| Less: Unamortized debt issuance costs | ( | ||||
| Less: Current maturities of long-term debt | ( | ||||
| Long-term debt | $ | ||||
9.Leases
The Company leases its operating facilities in Novato, California, under a non-cancelable operating lease through May 31, 2027. There are no options or rights to extend the term of this lease.
F-30
The following table reflects the Company’s ROU assets and lease liabilities as of December 31, 2024 and 2023:
| December 31, 2024 | December 31, 2023 | ||||||||||
| Assets: | |||||||||||
| Operating lease ROU assets, net | $ | $ | |||||||||
| Liabilities: | |||||||||||
| Operating lease liabilities, current | $ | $ | |||||||||
| Operating lease liabilities | |||||||||||
| $ | $ | ||||||||||
The following table presents supplemental cash flow information related to the Company’s operating leases for the year ended:
| Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
| Operating cash flows from operating leases | $ | $ | ||||||||||||
As of December 31, 2024, the maturity of operating lease liabilities was as follows:
| Year ending December 31: | |||||
| 2025 | $ | ||||
| 2026 | |||||
| 2027 | |||||
| Total payments | |||||
| Less: Interest | ( | ||||
| Present value of obligations | $ | ||||
The operating lease expense for the years ended December 31, 2024 and 2023 was $454,780 and $453,889 , respectively, of which $21,915 and $21,024 , respectively, were related to leases with a term of less than 12 months.
10.Contingencies
Litigation
The Company is subject to occasional lawsuits, investigations, and claims arising out of the normal conduct of business. As of the date the consolidated financial statements were available to be issued, management is not aware of any pending claims that will have a material impact on the Company’s consolidated financial statements.
11.Stockholders’ Deficit
Common Stock
The Company's common stock trades on the OTCQB Venture Market tier under the symbol “QTIH”. Pursuant to the terms of the Amended and Restated Certificate of Incorporation, the Company is authorized and has available for issuance 500,000,000 shares of common stock. Immediately following the Merger, there were 21,437,216 shares of
F-31
common stock outstanding with a par value of $0.0001 . The holder of each share of common stock is entitled to one vote.
The Company retroactively adjusted the shares issued and outstanding prior to March 4, 2024 to give effect to the exchange ratio established in the Business Combination Agreement to determine the number of shares of common stock into which they were converted.
Common stock reserved for future issuance as of December 31, 2024 is as follows:
| Common stock warrants | |||||
| Potential shares from Pre-Paid Advance | |||||
| Merger earnout consideration shares | |||||
| Options outstanding under the 2024 Incentive Plan | |||||
| Options available under the 2024 Incentive Plan | |||||
| Potential shares from Cable Car Loan | |||||
| Potential shares from convertible notes | |||||
Preferred Stock
The Company is authorized to issue 10,000,000 shares of preferred stock, with a par value of $0.0001 , with such designations, voting and other rights and preferences as may be determined from time to time by the Board of Directors. As of December 31, 2024 and 2023, there were no
QT Imaging Private Placement Warrants
In November 2022, the Company initiated an offering to sell to a select group of accredited investors only, on a private placement basis, 342,703 units for a purchase price of $11.67 per unit (the “Units”), each Unit consisting of one share of common stock and one warrant to purchase one share of common stock (the “QT Imaging Private Placement Warrants”) with an exercise price of $11.67 (the “2022 Offering”). From November 2022 to December 31, 2023, the Company has issued 167,925 Units for net proceeds of $1,932,850 , which 89,532 Units were issued during the year ended December 31, 2023 for total net proceeds of $1,026,550 . There were no Units issued during the year ended December 31, 2024. On March 4, 2024, all outstanding QT Imaging Private Placement Warrants were deemed out of the money and terminated in accordance with the Business Combination Agreement.
QT Imaging Warrants for Common Stock
In addition to the warrants sold as part of the Units in the 2022 Offering, the Company also issued warrants to consultants and to placement agents in association with debt issuances and past private offerings. At the option of the warrant holders, the warrants can be fully settled in shares of common stock, or converted via net share settlement, in which the warrant holder will receive shares equal to the number of shares purchasable under the warrants multiplied by the difference between the fair market value of the shares and the exercise price, divided by the fair market value of the shares.
F-32
The following table represents the QT Imaging warrant activity as follows for the year ended December 31, 2024:
| Number of Warrants | |||||
Outstanding, January 1, 2024 | |||||
| Exercised | ( | ||||
| Terminated pursuant to business combination agreement | ( | ||||
Outstanding, December 31, 2024 | |||||
The fair value of the QT Imaging warrants issued as part of the 2022 Offering and included in stockholders’ deficit in the consolidated balance sheets was $462,413 for the year ended December 31, 2023. The fair value of the remaining warrant granted during the year ended December 31, 2023 was $15,317 and was recorded as issuance costs against the proceeds received from the 2022 Offering. There were no QT Imaging warrants issued during the year ended December 31, 2024.
On March 4, 2024 and in accordance with the terms of the Business Combination Agreement, the Company cancelled and terminated all outstanding warrants that were deemed out of the money with an exercise price of or above $11.67 per warrant, including all warrants sold as part of the Units in the 2022 Offering and warrants that were issued to consultants and placement agents in association with debt issuances and past private offerings.
Warrants (Public Warrants, Private Placement Warrants, Working Capital Note Warrants, and PIPE Warrants)
Warrants will be exercisable at $11.50 per share, and pursuant to the terms of the warrant agreement governing such warrants (the “Warrant Agreement”), the exercise price and number of warrant shares issuable on exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary dividend or recapitalization, reorganization, merger or consolidation of the Company. In addition, if (x) the Company issues additional shares of common stock or equity-linked securities for capital raising purposes in connection with the closing of its initial Business Combination at an issue price or effective issue price of less than $9.20 per share of common stock (with such issue price or effective issue price to be determined in good faith by the Company’s Board of Directors, and in the case of any such issuance to the Company’s Founder or its affiliates, without taking into account any Founder Shares held by it prior to such issuance), (y) the aggregate gross proceeds from such issuances represent more than 65 % of the total equity proceeds, and interest thereon, available for the funding of the Company’s initial Business Combination on the date of the consummation of its initial Business Combination (net of redemptions), and (z) the VWAP of the Company’s common stock during the 20 trading-day period starting on the trading day prior to the day on which the Company consummates its initial Business Combination (such price, the “Market Value”) is below $9.20 per share, the exercise price of the warrants will be adjusted (to the nearest cent) to be equal to 115 % of the greater of (i) the Market Value or (ii) the price at which the Company issues the additional shares of common stock or equity-linked securities.
Each warrant will become exercisable on the later of 30 days after the completion of the Merger and will expire five years after the completion of the Merger. If the Company is unable to deliver registered shares of common stock to the holder upon exercise of the warrants during the exercise period, there will be no net cash settlement of these warrants and the warrants will expire worthless, unless they may be exercised on a cashless basis in the circumstances described in the Warrant Agreement. Once the warrants become exercisable, the Company may redeem the outstanding warrants in whole and not in part at a price of $0.01 per warrant upon a minimum of 30 days’ prior written notice of redemption, only in the event that the last sale price of the Company’s shares of common stock equals or exceeds $18.00 per share for any 20 trading days within the 30 -trading day period ending on the third trading day before the Company sends the notice of redemption to the warrant holders.
Under the terms of the Warrant Agreement, the Company has agreed to use its best efforts to file a new registration statement under the Securities Act of 1933, as amended (the “Securities Act”), following the completion of the Merger, for the registration of the shares of common stock issuable upon exercise of the warrants included in the public units issued in the Company’s initial public offering (the “Public Units”), the private placement units undertaken by the Company concurrently with its initial public offering (the “Private Placement Units”) and the private placement units that were issued upon conversion of working capital notes issued by the Company prior to the
F-33
Merger, which conversion occurred concurrent with the Merger. The new registration statement was filed on April 1, 2024, and was declared effective by the SEC on May 22, 2024.
As of December 31, 2024, there were 23,889,364 warrants outstanding from those that were initially included as a constituent security of the Public Units and the Private Placement Units (the “PubCo Warrants”) with an exercise price of $11.50 per warrant and expiring on March 4, 2029. On May 13, 2024, the exercise price of PubCo Warrants was reduced from $11.50 to $2.30 per warrant and the price per share related to the redemption events described above decreased from $18.00 per share to $3.60 per share in accordance with the terms of the Warrant Agreement as discussed above. The modification in exercise price related to the Public Warrants, which are equity classified, was accounted as a deemed dividend, which resulted in an adjustment of $5,185,502 to accumulated deficit during the year ended December 31, 2024. The effect of the modification in exercise price related to the Private Placement Warrants and Working Capital Note Warrants, which are liability classified, was recorded in other expense, net within the consolidated statements of operations and comprehensive loss and amounted to $200,513 during the year ended December 31, 2024.
On November 22, 2024, the Company completed a private placement with related parties (the “Private Placement”), pursuant to the terms and conditions of a securities purchase agreement (the “Securities Purchase Agreement”). At the closing of the Private Placement, the Company issued warrants (the “PIPE Warrants”) to purchase up to 4,383,558 shares of common stock that are issuable upon its exercise. Each PIPE Warrant sold in the Private Placement is exercisable for one share of common stock at an exercise price of $0.672 per share, and is exercisable beginning on May 22, 2025 and ending on May 22, 2030. As of December 31, 2024, there were 4,383,558 PIPE Warrants outstanding.
12.Stock Incentive Plans
2024 Equity Incentive Plan
On February 15, 2024, at the Annual Meeting, the GigCapital5 stockholders considered and approved the 2024 Equity Incentive Plan (the “2024 Incentive Plan”) and reserved 2,358,093 shares of common stock for issuance thereunder. The 2024 Incentive Plan became effective immediately upon the Closing of the Business Combination on March 4, 2024. The term of the 2024 Incentive Plan is 10 years. The number of shares of common stock reserved for issuance under the 2024 Incentive Plan will automatically increase on January 1 of each year, beginning on January 1, 2025 and continuing through January 1, 2035, by 5 % of the total number of shares of common stock outstanding on December 31 of the preceding calendar year, or a lesser number of shares as may be determined by the Board of Directors. Under the 2024 Incentive Plan, the Company may issue stock options, stock appreciation rights (“SARs”), restricted stock awards (“RSAs”), restricted stock units (“RSUs”), and performance awards (“PAs”). The term of stock options may not exceed 10 years and is subject to vesting conditions, which is subject to the option holder’s continued service to the Company. The exercise price of any stock option award cannot be less than fair market value of the Company’s common stock, provided, however, that an incentive stock option granted to an employee who owns more than 10 % of the total combined voting power of all classes of stock of the Company or any parent or subsidiary, must have an exercise price of no less than 110 % of the fair market value of the Company’s common stock and a term that does not exceed five years .
There were 2,219,000 options outstanding under the 2024 Incentive Plan as of December 31, 2024.
QT Imaging Incentive Plan
In September 2021, the Board of Directors approved and the Company adopted the Plan (the “QT Imaging Plan”). The maximum aggregate number of shares of common stock that the Company may award under the QT Imaging Plan was 7,000,000 . The term of the QT Imaging Plan was originally 10 years. The QT Imaging Plan was administered by the compensation committee of the Company’s Board of Directors (the “Administrator”). The Company may grant awards to eligible participants which may take the form of stock options (both incentive stock options and non-qualified stock options), stock purchase rights, restricted stock, restricted stock units and performance stock awards. Awards may be granted to employees, directors, and consultants (as defined in the QT Imaging Plan.) The term of any stock option award may not exceed 10 years and may be subject to vesting conditions, as determined by the Administrator.
F-34
Incentive stock options may only be granted to employees of the Company or any subsidiary that is a “subsidiary corporation” within the meaning of Section 424(f) of the Internal Revenue Code. The exercise price of any stock option award cannot be less than fair market value of the Company’s common stock, provided, however, that an incentive stock option granted to an employee who owns more than 10 % of the total combined voting power of all classes of stock of the Company or any parent or subsidiary, must have an exercise price of no less than 110 % of the fair market value of the Company’s common stock and a term that does not exceed five years . Vesting is subject to the option holder’s continued service to the Company, ranging up to a four-year period. Unvested options are subject to forfeiture upon termination of employment. On March 4, 2024, the QT Imaging Plan was terminated in accordance with the terms of the Business Combination Agreement and the options to purchase 1,237,681 shares of common stock were cancelled at the close of the Business Combination in accordance with the terms of the Business Combination Agreement.
The following table summarizes information regarding activity in the QT Imaging Plan and the 2024 Incentive Plan during the year ended December 31, 2024:
| Number of Options | Weighted- Average Exercise Price | Weighted-Average Remaining Contractual Life (years) | |||||||||||||||
Outstanding, January 1, 2024 | $ | ||||||||||||||||
| Granted under the 2024 Incentive Plan | $ | ||||||||||||||||
| Cancelled | ( | $ | |||||||||||||||
| Terminated pursuant to Business Combination Agreement | ( | $ | |||||||||||||||
Outstanding, December 31, 2024 | $ | ||||||||||||||||
Exercisable as of December 31, 2024 | $ | — | |||||||||||||||
Vested and expected to vest as of December 31, 2024 | $ | ||||||||||||||||
During the year ended December 31, 2024, a total of 2,299,000 0.46 no
The determination of the fair value of options granted during the year ended December 31, 2024 is computed using the Black-Scholes option pricing model with the following weighted-average assumptions:
| Stock price per share | $ | ||||
| Expected option term (years) | |||||
| Expected volatility | % | ||||
| Risk-free rate of return | % | ||||
| Expected annual dividend yield | % | ||||
Option pricing models require the input of various subjective assumptions, including the option’s expected life and the price volatility of the underlying stock. The expected stock price volatility is based on the analysis of volatilities of the Company’s selected public peer group over a period commensurate with the expected term of the options. The expected term of employee options represents the weighted-average period the options are expected to remain outstanding and was derived using the simplified method for awards that qualify for its “plain-vanilla” options. All awards that are outstanding are qualified for “plain-vanilla” options. The risk-free interest rate is based on the U.S. Treasury interest rates whose term in consistent with the expected life of the stock options. No dividend yield is included as the Company has not issued any dividends and do not anticipate issuing any dividends in the future.
F-35
The following table shows stock-based compensation expense by functional area in the consolidated statements of operations and comprehensive loss for the years ended December 31, 2024 and 2023:
| Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
| Research and development | $ | $ | ||||||||||||
| Selling, general and administrative | ||||||||||||||
| $ | $ | |||||||||||||
As of December 31, 2024, the total unrecognized compensation cost related to all nonvested stock options was $758,294 and the weighted-average period over which it is expected to be recognized is 2.1 years.
13.National Institutes of Health Subaward
On August 18, 2022, the Company was awarded a grant of up to $1,078,347 as a subaward through the Board of Trustees of the University of Illinois for the purpose of developing a quantitative ultrasound breast scanner for identifying early response of breast cancer to chemotherapy. The grant is a cost reimbursement subaward that is allocated annually over five years , subject to the availability of funds and satisfactory progress of the project. The award expires July 31, 2027 and may be terminated by either party with 30 days written notice. Any grant proceeds received do not require repayment. As of December 31, 2024, the Company incurred total costs of $388,359 against the year one allocation of $351,994 , against the year two allocation of $194,566 , and against the year three allocation of $119,000 . During the year ended December 31, 2024, the Company incurred costs of $39,305 , of which $23,509 of grant income was recognized as an offset to research and development expense and $15,796 was recognized as an offset to selling, general and administrative expense in the consolidated statements of operations and comprehensive loss. During the year ended December 31, 2023, the Company incurred costs of $318,276 , of which $277,037 of grant income was recognized as an offset to research and development expense and $41,239 was recognized as an offset to selling, general and administrative expense in the consolidated statements of operations and comprehensive loss. As of December 31, 2024 and 2023, the grant receivable was $11,255 and $161,638 , respectively, and is included in prepaid expenses and other current assets on the consolidated balance sheets.
14.Income Taxes
Loss before income tax expense (benefit) consisted of the following for the years ended December 31:
| 2024 | 2023 | ||||||||||
| United States | $ | ( | $ | ( | |||||||
| International | |||||||||||
Total loss before income tax expense (benefit) | $ | ( | $ | ( | |||||||
F-36
Income tax expense (benefit) consisted of the following for the years ended December 31:
| 2024 | 2023 | ||||||||||
| Current: | |||||||||||
| Federal | $ | ( | $ | ||||||||
| State | ( | ||||||||||
| Foreign | |||||||||||
Total current tax expense (benefit) | ( | ||||||||||
| Deferred: | |||||||||||
| Federal | |||||||||||
| State | |||||||||||
| Foreign | |||||||||||
| Total deferred tax expense | |||||||||||
Total income tax expense (benefit) | $ | ( | $ | ||||||||
Income tax expense (benefit) differed from the amount computed by applying the federal statutory income tax rate to pretax loss as a result of the following for the years ended December 31:
| 2024 | 2023 | ||||||||||
| Federal tax at statutory rate | $ | ( | $ | ( | |||||||
| State taxes | ( | ( | |||||||||
| Change in valuation allowance | |||||||||||
Acquired intangibles | ( | ||||||||||
| Nondeductible | ( | ||||||||||
| Other | |||||||||||
Total income tax expense (benefit) | $ | ( | $ | ||||||||
The tax effects of temporary differences that give rise to the Company’s deferred tax assets and liabilities are related to the following as of December 31:
| 2024 | 2023 | ||||||||||
| Deferred tax assets: | |||||||||||
| Net operating losses | $ | $ | |||||||||
| Stock-based compensation | |||||||||||
| Operating lease liabilities | |||||||||||
| Section 174 expenses, net | |||||||||||
| Accruals and reserves | |||||||||||
| Intangible assets | |||||||||||
| Property and equipment | |||||||||||
| Gross deferred tax assets | |||||||||||
| Valuation allowance | ( | ( | |||||||||
| Net deferred tax assets | |||||||||||
| Deferred tax liabilities: | |||||||||||
| Operating lease right-of-use assets | ( | ( | |||||||||
| Net deferred tax assets (liabilities) | $ | $ | |||||||||
As of December 31, 2024, based on the Company’s recent history of losses and its forecasted losses, management believes on the more-likely-than-not basis that a full valuation allowance is required. Accordingly, the Company provided a full valuation allowance on its federal and state deferred tax assets. During the years ended December 31,
F-37
2024 and 2023, the valuation allowance increased by $5,861,012 and $1,080,617 , respectively. As of December 31, 2024, the Company had federal and state net operating loss (“NOL”) carryforwards of $19,790,000 and $14,375,000 respectively. The federal NOL will not expire and the state NOL will begin to expire in 2040.
A reconciliation of the beginning and ending amount of gross unrecognized tax benefits is a follows as of December 31:
| 2024 | 2023 | ||||||||||
| Balance as the beginning of the year | $ | $ | |||||||||
| Increases related to prior year tax positions | |||||||||||
| Increases related to current year tax positions | |||||||||||
| Balance as the end of the year | $ | $ | |||||||||
The unrecognized tax benefits for the year ended December 31, 2024, if recognized, would not affect the effective income tax rate due to the valuation allowance that currently offsets the deferred tax assets. It is reasonably possible that the unrecognized tax benefits balance will change within twelve months by a range of zero to $49,255 due to the Company’s intent to file a tax accounting method change.
15.Related Party Transactions
Convertible Notes Payable
In July 2020, the Company issued three convertible notes to three of its stockholders for advances up to $3,500,000 in principal (the “2020 Notes”) and bearing annual interest of 5 % on any amounts drawn. An additional note was issued in March 2022 as part of the 2020 Notes, but with an annual interest rate of 8 %. All principal and interest payments are due on or before July 1, 2025. The 2020 Notes are convertible, at the holder’s option, into shares of common stock of the Company at the lower of $14.59 per share or the offering price in a financing of at least $5,000,000 in equity from unaffiliated parties. As of December 31, 2024, an aggregate of 253,199 shares of common stock would be issued if the entire principal and interest under the 2020 Notes was converted. Management assessed whether the embedded features in the 2020 Notes should have been bifurcated from the debt host and concluded that none of the features were required to be accounted for separately from the debt instruments.
As of December 31, 2024 and 2023, the outstanding amount of the 2020 Notes was $3,143,725 550,430 and $377,772 , respectively.
Working Capital Loan and Extension Note
On May 3, 2023, the Company issued a promissory note (the “Working Capital Note”) to a stockholder for a principal amount of $250,000 . The Working Capital Note was subsequently amended and restated six times on June 12, 2023 to add an additional principal amount of $100,000 , August 15, 2023 to add an additional principal amount of $75,000 , August 29, 2023 to add an additional principal amount of $100,000 , September 12, 2023 to add an additional principal amount of $75,000 , September 15, 2023 to add an additional principal amount of $50,000 , and October 26, 2023 to add an additional principal amount of $55,000 , for an aggregate principal amount outstanding as of December 31, 2024 under the Working Capital Note of $705,000 . The Working Capital Note was issued to provide the Company with additional working capital during the period prior to consummation of the Business Combination Agreement with GigCapital5. The Working Capital Note is interest-free and originally matured on the earlier of (i) the date on which the Company consummated the Business Combination with GigCapital5; (ii) the date the Company winds up; or (iii) December 31, 2023. The Working Capital Note may be prepaid without penalty. On March 4, 2024, the holder of the Working Capital Note agreed to extend and subordinate the promissory note pursuant to and in accordance with the terms of the Business Combination Agreement. Effective on the Closing of the Business Combination, the Working Capital Note cannot be repaid prior to the repayment or conversion of the Yorkville Note received from Yorkville (see Note 8).
F-38
On March 4, 2024, the Company assumed the $1,560,000 outstanding debt balance due to a related party (the “Extension Note”) pursuant to the Business Combination Agreement. The Extension Note did not bear any interest and could not be repaid prior to the repayment of the Yorkville Note received from Yorkville. On November 22, 2024, the Extension Note was cancelled in its entirety in exchange for the purchase of 2,671,232 shares of common stock at a purchase price of $0.584 per share and issuance of 2,671,232 of PIPE Warrants with exercise price of $0.672 per share.
Private Placement
On November 12, 2024, the Company and certain board members entered into the Securities Purchase Agreement for the issuance of shares of common stock plus warrants for the purchase of common stock with an aggregate purchase price of $999,998 in exchange for 1,712,326 shares of common stock at an issuance price of $0.584 per share and 1,712,326 of PIPE Warrants with an exercise price of $0.672 per share, the closing of which occured on November 22, 2024.
Management Services and Business Associate Agreement
In September 2020, QT Imaging entered into a Management Services Agreement (the “Agreement”) and a Business Associate Agreement with John C. Klock, M.D., a California sole proprietorship operating as the QT Imaging Center (the “Practice”). John C. Klock, M.D. was the Chief Executive Officer of QT Imaging, serves on its Board of Directors, and was the largest single stockholder of QT Imaging. The Practice provided medical imaging to patients using the QT Breast Scanner. Under the terms of the Agreement, the Company agreed to provide business services to the Practice including use of the facility which formerly operated as the Marin Breast Health Trial Center, including furniture and medical equipment, as well as use of certain personnel. In exchange for those services, the Practice agreed to pay the Company a management fee. Fees paid to QT Imaging during the years ended December 31, 2024 and 2023 were $12,000 and $48,000 , respectively. These fees were recorded as a reduction to selling, general and administrative expenses on the consolidated statements of operations and comprehensive loss. During the years ended December 31, 2024 and 2023, there were no significant purchases made by the Practice. As of December 31, 2024 and 2023, there were no significant amounts due from the Practice. This Agreement was terminated and replaced by the Space and Equipment Sublease Agreement on April 17, 2024 and Services Agreement on April 5, 2024.
Services Agreement
On April 5, 2024, the Company entered into that certain Services Agreement (the “Services Agreement”) with the Practice dated as of April 1, 2024 pursuant to which the Practice agreed to provide its services to the Company, including but not limited to providing healthcare services to patients, assisting with clinical trials and studies and assisting with drafting of institutional review board approved clinical protocols, assisting with the performance of research and development activities on behalf of the Company, providing comprehensive multi-day training on the operation of breast imaging technology for radiologist customers and other customer staff such as technicians, performing clinical validation of imaging software changes which may include recruiting patients, training personnel on the operation of the Company’s imaging technology, as well as other services as specified in the Services Agreement. The term of the Services Agreement is one year unless earlier terminated and shall auto-renew for successive -year periods, unless otherwise terminated. During the year ended December 31, 2024, the Company incurred $55,971 in accordance with the Services Agreement. As a result of Dr. Klock's retirement on December 31, 2024, the Services Agreement was terminated.
Space and Equipment Sublease Agreement
On April 17, 2024, the Company entered into a Space and Equipment Sublease Agreement (the “Space and Equipment Sublease”) with the Practice, pursuant to which the Practice will sublease certain medical equipment and space, currently leased from Hamilton Landing Novato LLC by the Company, to the Practice for use in its operations, on a full-time and exclusive basis. The Practice shall pay to the Company a $5,666 rental fee (the “Rent”) for the Subleased Space (as defined in the Space and Equipment Sublease) on a monthly basis, payable on the first day of each month and no later than ten days thereafter, with the Rent to be pro-rated for any partial month. The parties have determined that the Rent equals the fair market value of the Subleased Space and Subleased Equipment (as defined in the Space and Equipment Sublease), without taking into account the proximity of the parties or the space to any source, volume
F-39
or value of referrals between the parties or any patient thereof. Further, the Practice shall pay when due all sales, use, personal property, leasing, excise or other fees, taxes, charges or withholdings of any kind imposed against the Company, the Practice or the Subleased Equipment with respect to the Space and Equipment Sublease, the Subleased Equipment, or any related fees, receipts or earnings, including local taxes and personal property taxes. The term of the Space and Equipment Sublease is one year unless terminated and shall auto-renew for successive -year periods, unless otherwise terminated. During the year ended December 31, 2024, the Company recorded $50,994 of sublease income in other expense, net within the consolidated statements of operations and comprehensive loss. As a result of Dr. Klock's retirement on December 31, 2024, the Space and Equipment Sublease Agreement was terminated.
Deferred Revenue
In July 2023, an order was placed and a downpayment of $200,000 was made for a breast imaging system by 303 Development Corporation (the “Foundation”). The executive director of the Foundation is a current investor and a was a previous board member of the Company. In December 2023, an additional $100,000 was paid towards the purchase. In June 2024, the Company cancelled this order and refunded the full deposit of $300,000 to the related party. As of December 31, 2024 and 2023, the Company had a deferred revenue balance of zero and $300,000 , respectively, related to this order.
16. Segment Information
The Company has one
The following table presents information about reported segment revenue, significant segment expenses, and segment net loss for the years ended December 31, 2024 and 2023:
| Year Ended December 31, | ||||||||||||||
| 2024 | 2023 | |||||||||||||
| Revenue | $ | $ | ||||||||||||
| Less: | ||||||||||||||
| Cost of revenue | ||||||||||||||
Research and development1 | ||||||||||||||
Selling, general and administrative1 | ||||||||||||||
| Other expense, net | ||||||||||||||
| Change in fair value of warrant liability | ( | |||||||||||||
| Change in fair value of derivative liability | ( | |||||||||||||
| Change in fair value of earnout liability | ( | |||||||||||||
| Interest expense, net | ||||||||||||||
| Income tax expense (benefit) | ( | |||||||||||||
Consolidated net loss | $ | ( | $ | ( | ||||||||||
Refer to Note 1 for segment information related to revenue.
17. Revised Financial Statements
F-40
The Company has revised its condensed consolidated financial statements as of and for the three months ended March 31, 2024, as of and for the six months ended June 30, 2024 and as of and for the nine months ended September 30, 2024 to correct a misstatement identified related to the initial recognition of the earnout liability, which is described in Notes 2 and 3. The Company assessed the materiality of this misstatement in accordance with Securities and Exchange Commission Staff Accounting Bulletin No. 108 – “Quantifying Misstatements” and concluded this error was not qualitatively material. As such, the correction of the error for the affected periods will be reflected prospectively in the Quarterly Reports on Form 10-Q for fiscal year 2025.
The effects of this revision on the applicable line items within the condensed consolidated financial statements were as follows:
Condensed Consolidated Balance Sheet Line Items Impacted | March 31, 2024 - As Filed | Adjustments | March 31, 2024 - As Revised | ||||||||||||||
Additional paid-in capital | $ | $ | ( | $ | |||||||||||||
| Accumulated deficit | $ | ( | $ | $ | ( | ||||||||||||
| Other Condensed Consolidated Financial Statement Line Items | Three Months Ended March 31, 2024 - As Filed | Adjustments | Three Months Ended March 31, 2024 - As Revised | ||||||||||||||
Condensed Consolidated Statement of Operations and Comprehensive Loss | |||||||||||||||||
| Change in fair value of earnout liability | $ | ( | $ | $ | |||||||||||||
| Net loss and comprehensive loss | $ | ( | $ | $ | ( | ||||||||||||
| Net loss per share - basic and diluted | $ | ( | $ | $ | ( | ||||||||||||
Condensed Consolidated Statement of Cash Flows | |||||||||||||||||
| Net loss | $ | ( | $ | $ | ( | ||||||||||||
| Change in fair value of earnout liability | $ | $ | ( | $ | ( | ||||||||||||
Condensed Consolidated Balance Sheet Line Items Impacted | June 30, 2024 - As Filed | Adjustments | June 30, 2024 - As Revised | ||||||||||||||
Additional paid-in capital | $ | $ | ( | $ | |||||||||||||
| Accumulated deficit | $ | ( | $ | $ | ( | ||||||||||||
| Other Condensed Consolidated Financial Statement Line Items | Six Months Ended June 30, 2024 - As Filed | Adjustments | Six Months Ended June 30, 2024 - As Revised | ||||||||||||||
Condensed Consolidated Statement of Operations and Comprehensive Loss | |||||||||||||||||
| Change in fair value of earnout liability | $ | ( | $ | $ | |||||||||||||
| Net loss and comprehensive loss attributable to QT Imaging Holdings, Inc. | $ | ( | $ | $ | ( | ||||||||||||
| Net loss and comprehensive loss attributable to common stockholders | $ | ( | $ | $ | ( | ||||||||||||
| Net loss per share - basic and diluted | $ | ( | $ | $ | ( | ||||||||||||
Condensed Consolidated Statement of Cash Flows | |||||||||||||||||
F-41
| Net loss | $ | ( | $ | $ | ( | ||||||||||||
| Change in fair value of earnout liability | $ | $ | ( | $ | ( | ||||||||||||
Condensed Consolidated Balance Sheet Line Items Impacted | September 30, 2024 - As Filed | Adjustments | September 30, 2024 - As Revised | ||||||||||||||
Additional paid-in capital | $ | $ | ( | $ | |||||||||||||
| Accumulated deficit | $ | ( | $ | $ | ( | ||||||||||||
| Other Condensed Consolidated Financial Statement Line Items | Nine Months Ended September 30, 2024 - As Filed | Adjustments | Nine Months Ended September 30, 2024 - As Revised | ||||||||||||||
Condensed Consolidated Statement of Operations and Comprehensive Loss | |||||||||||||||||
| Change in fair value of earnout liability | $ | ( | $ | $ | |||||||||||||
| Net loss and comprehensive loss attributable to QT Imaging Holdings, Inc. | $ | ( | $ | $ | ( | ||||||||||||
| Net loss and comprehensive loss attributable to common stockholders | $ | ( | $ | $ | ( | ||||||||||||
| Net loss per share - basic and diluted | $ | ( | $ | $ | ( | ||||||||||||
Condensed Consolidated Statement of Cash Flows | |||||||||||||||||
| Net loss | $ | ( | $ | $ | ( | ||||||||||||
| Change in fair value of earnout liability | $ | $ | ( | $ | ( | ||||||||||||
18. Subsequent Events
On January 9, 2025, the Company and Yorkville entered into the Third Omnibus Amendment to the Yorkville Note, (the “Third Amendment”), pursuant to which, the Company and Yorkville agreed that for $1.5 million of the then current outstanding balance due under the Yorkville Note (principal and unpaid accrued interest), the fixed price for conversion shall be modified to $0.584 per share, and for the remainder of the balance, the fixed price shall not be changed but shall remain $4.61395 per share as provided for in the Yorkville Note when the Company issued it on March 4, 2024. Further, the Third Amendment removed the Company’s obligation to make monthly payments to Yorkville, previously owing due to the occurrence of the Trigger Event, such that no further monthly payments will be owed during the period beginning on the date of the Third Amendment and ending on the maturity date of the Yorkville Note of March 31, 2026. In exchange for this relief, the aggregate purchase price owed to the Company from the first Advance that occurs pursuant to the terms of the SEPA (the “Advance Proceeds”) shall be paid by Yorkville offsetting the amount of the Advance Proceeds against an equal amount outstanding under the Yorkville Note (first towards accrued and unpaid interest, and then towards outstanding principal and the corresponding payment premium in respect of such principal amount, if applicable), and that for any subsequent Advances pursuant to the terms of the SEPA, Yorkville shall pay half of such Advance Proceeds directly to the Company and the other half of such Advance Proceeds shall be paid by Yorkville offsetting the amount of the Advance Proceeds against an equal amount outstanding under the Yorkville Note (first towards accrued and unpaid interest, and then towards outstanding principal and the corresponding payment premium in respect of such principal amount, if applicable). On January 9, 2025, the Company delivered its first Advance Notice under the SEPA for the sale of 885,000 shares of common stock. This resulted in the reduction of an additional $182,682 in principal of the Yorkville Note.
On January 9, 2025, the Company and Cable Car entered into an Omnibus Amendment (the “Cable Car Amendment”) to amend certain terms of the Cable Car Note, including a reduction of the conversion price for the Cable Car Note to $0.584 per share. Further, the Cable Car Amendment provides that the maturity date for the Cable Car Note shall be extended to March 31, 2026, in consideration for which, the Company shall pay an extension fee (the “Extension Fee”) of $90,000 to Cable Car, with such fee being added to the amount due and payable on such maturity date, unless the
F-42
Cable Car Note is earlier converted pursuant to its terms, in which event the Extension Fee shall also be converted. No interest shall accrue or be due on the Extension Fee. Pursuant to the Cable Car Amendment, interest shall accrue on the outstanding principal balance of the Cable Car Note at an annual rate equal to 6 %, with interest being calculated based on a 365-day year and the actual number of days elapsed, to the extent permitted by applicable law. Interest shall be due and payable on the maturity date for the Cable Car Note, unless the Cable Car Note is earlier converted pursuant to its terms, in which event such accrued and unpaid interest shall also be converted. In addition, in connection with any sale, assignment, transfer, or other disposition (a “Cable Car Sale”) of any shares into which the Cable Car Note is converted pursuant to its terms, the Cable Car Amendment provides that to the extent such Cable Car Sale is made pursuant to Rule 144, provided that Rule 144 is available as an exemption from the registration requirements for such Cable Car Sale, if requested by Cable Car and upon delivery by Cable Car of such customary representations and other documentation reasonably acceptable to the Company in connection with transactions relying upon Rule 144, the Company shall use commercially reasonable efforts to cause its transfer agent to remove any restrictive legends related to the book entry account holding such shares sold or disposed of by Cable Car without restrictive legends within two business days of such request.
On January 23, 2025, the Company entered into a Sublease Agreement (the “Sublease”) with the Practice, pursuant to which the Practice will sublease certain space, currently leased from Hamilton Landing Novato LLC by the Company pursuant to the “Prime Lease” (as defined in the Sublease), to the Practice for use in its operations, on a full-time and exclusive basis. The Practice shall pay to the Company a monthly rental fee for the Subleased Space (as defined in the Sublease) in an amount equal to $5,666 until May 31, 2025, with such amount increasing to $5,836 during the period from June 1, 2025 until May 31, 2026, and subsequently $6,011 during the period from June 1, 2026 until May 31, 2027. The term of the Sublease is one year unless terminated and shall auto-renew on a month-to-month basis thereafter, unless otherwise terminated. The Sublease shall expire automatically upon the termination of the Prime Lease, which is set to terminate in May 2027.
On February 26, 2025, the Company entered into a credit agreement (the “Credit Agreement”) that provides a senior secured term loan (the “Lynrock Lake Term Loan”) with Lynrock Lake Master Fund LP (“Lynrock Lake”), a related party. The Credit Agreement is secured by a first priority lien on substantially all assets of the Company and provides for a term loan in the aggregate principal amount of $10,100,000 at an interest rate of 10.0 % per annum, compounded quarterly. The maturity date of the Credit Agreement is March 31, 2027. Furthermore, in connection with the Lynrock Lake Term Loan, the Company issued to Lynrock Lake, pursuant to the terms of a Warrant to Purchase common stock (the “Lynrock Lake Warrant”), warrants to purchase 61,000,000 shares of its common stock at an exercise price of $0.40 per share. The Lynrock Lake Warrant is exercisable until February 26, 2035. Lynrock Lake may cashless exercise the Lynrock Lake Warrant. The Lynrock Lake Warrant is also subject to anti-dilution adjustments to the exercise price and the number of shares which may be purchased upon exercise of the Lynrock Lake Warrant in the event that the Company issues shares of common stock (or derivative securities) at a price that is either less than the $0.40 exercise price or the fair market value of a share of common stock from the immediately prior trading day.
On February 26, 2025, the Company used a portion of the proceeds of the Lynrock Lake Term Loan to pay Yorkville an amount equal to $3,000,000 in cash and issued to Yorkville warrants to purchase 15,000,000 shares of its common stock at an exercise price of $0.40 per share pursuant to a Warrant to Purchase common stock (the “Yorkville Warrant”) to fully settle and discharge the Company’s obligations under the Yorkville Note and extinguish the Yorkville Note as having been fully performed. The Yorkville Warrant is exercisable until February 26, 2030. Yorkville may cashless exercise the Yorkville Warrant. The Yorkville Warrant is also subject to adjustments in the event that the Company’s common stock undergoes a split, reverse-split or similar event. Furthermore, the Yorkville Warrant has provided the holder with piggyback registration rights. The Company and Yorkville also entered into that certain Termination Agreement, dated February 26, 2025 (the “Termination Agreement”), pursuant to which the parties acknowledged the termination of the SEPA effective February 26, 2025.
On February 26, 2025, the Company used a portion of the proceeds of the Lynrock Lake Term Loan to pay Cable Car an amount equal to the full principal, interest and fees amount of approximately $1,625,000 in cash to fully settle and discharge the Company’s obligations under the Cable Car Note and extinguish the Cable Car Note as having been fully performed.
In connection with the issuance of the Lynrock Lake Term Loan, on February 26, 2025, the maturity dates on both the Convertible Note Payable and Working Capital Notes above were extended to October 21, 2027.
F-43
On March 28, 2025, the Company entered into the Canon Manufacturing Agreement with Canon Medical Systems Corporation (“CMSC”). Pursuant to the terms of the Canon Manufacturing Agreement, the Company appoints CMSC as the exclusive manufacturer of the QT Breast Scanners to be distributed by NXC. The purchase prices applicable to the purchase orders as of the date of the Canon Manufacturing Agreement shall be separately agreed between the parties in writing. The term of the Canon Manufacturing Agreement is through December 31, 2026.
F-44
Item 9: Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A: Controls and Procedures
Disclosure Controls and Procedures
The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, refers to controls and other procedures that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Evaluation of Disclosure Controls and Procedures
As required by Rules 13a-15 and 15d-15 under the Exchange Act, our Chief Executive Officer and Chief Financial Officer carried out an evaluation of the effectiveness of our disclosure controls and procedures as of December 31, 2024. Based on their evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) were not effective due to the material weakness related to technical accounting described below.
Material Weaknesses Identified
In connection with the preparation of our consolidated financial statements as of and for the fiscal year ended December 31, 2023, we identified a material weakness in our internal control over financial reporting related to lack of segregation of duties around key accounting processes resulting from limited personnel resources.
In connection with the review of our condensed consolidated financial statements as of and for the three months ended March 31, 2024, we identified a material weakness in our internal control over financial reporting related to technical accounting aspects of certain material transactions.
In connection with the audit of the consolidated financial statements as of and for the fiscal year ended December 31, 2024, we identified a misstatement in the condensed consolidated financial statements for the three-month period ended March 31, 2024, six-month period ended June 30, 2024 and nine-month period ended September 30, 2024 as a result of the material weakness related to technical accounting. We have made significant progress in our remediation efforts, which included implementing technology, hiring personnel, and other activities, including engaging external resources and, as a result, have remediated the material weakness related to lack of segregation of duties. We expect to remediate the material weakness related to technical accounting in the first quarter of 2025.
Changes in Internal Control Over Financial Reporting
Other than the remediation efforts related to the material weaknesses as described above, there were no changes in our internal control over financial reporting that occurred in the three months ended December 31, 2024 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
The effectiveness of any system of internal control over financial reporting, including ours, is subject to inherent limitations, including the exercise of judgment in designing, implementing, operating, and evaluating the controls and procedures, and the inability to eliminate misconduct completely. Accordingly, in designing and evaluating the disclosure controls and procedures, management recognizes that any system of internal control over financial reporting, including ours, no matter how well designed and operated, can only provide reasonable, not absolute assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints, and that management is required to apply its judgment in evaluating the benefits of possible controls and procedures relative to their costs. Moreover, projections of any evaluation of effectiveness to future periods are subject
111
to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. We intend to continue to monitor and upgrade our internal controls as necessary or appropriate for our business but cannot assure you that such improvements will be sufficient to provide us with effective internal control over financial reporting.
Item 9B: Other Information
Item 9C: Disclosure Regarding Foreign Jurisdictions that Prevent Inspection
Not Applicable.
112
Part III
Item 10: Directors, Executive Officers and Corporate Governance
The following is a list of the persons who currently serve, as of the date of this annual report, as directors and executive officers of QT Imaging Holdings.
| Name | Age | Position | ||||||||||||
| Dr. Raluca Dinu | 51 | Chief Executive Officer and Director (Class III) | ||||||||||||
Anastas Budagov | 37 | Chief Financial Officer | ||||||||||||
Dr. Avi Katz (4) | 66 | Chairman of the Board of Directors (Class III) | ||||||||||||
| Dr. John C. Klock | 80 | Director (Class III) | ||||||||||||
Ross Taylor (1)(2)(3) | 61 | Director (Class II) | ||||||||||||
Daniel Dickson (2)(3) | 71 | Director (Class I) | ||||||||||||
James Greene (1)(2)(3) | 69 | Director (Class I) | ||||||||||||
Professor Zeev Weiner (1)(3) | 65 | Director (Class II) | ||||||||||||
__________________
(1)Member of the Audit Committee.
(2)Member of the Compensation Committee.
(3)Member of the Nominating and Corporate Governance committee.
(4)Chairman of the Board.
Executive Officers
Dr. Raluca Dinu, Chief Executive Officer and Director.
Dr. Raluca Dinu co-founded GigCapital5 with Dr. Avi S. Katz, who is our Chairman of the Board, and has served as a member of our Board, as well as our President, Chief Executive Officer and Secretary since February 2021. Dr. Dinu has spent approximately 21 years in international executive positions within the TMT industry working for privately held start-ups, middle-cap companies and large enterprises. In these roles, Dr. Dinu has been instrumental in launching and accelerating entities, building teams, large scale fund-raising, developing key alliances and technology partnerships, M&A activities, business development, financial management, global operations and sales and marketing. She served as the Chief Executive Officer of GigCapital2, Inc. (“GIG2”) from August 2019 to June 2021 and as a member of its board of directors since March 2019 and has continued in that role after that company became UpHealth, Inc. She also served on the board of directors of GigCapital3, Inc. (“GIG3”) beginning in February 2020 and continued in that role after that company became Lightning eMotors, Inc. in May 2021 until October 2021. She has also served as a member of the board of directors of BigBear.ai Holdings, Inc. since its inception in December 2020 as GigCapital4, Inc. (“GIG4”) until March 2024, and prior to the December 2021 business combination, was also the President, Chief Executive Officer and Secretary of GIG4 since its inception in December 2020. Drs. Katz and Dinu co-founded GigCapital7 Corp. (“GIG7”) in May 2024, a Private-to-Public Equity (PPE) company formed for the purpose of acquiring a company in the TMT, AI/ML, cybersecurity, MedTech, semiconductor and sustainable industries, and Dr. Dinu has served on the board of directors of Gig7 since its inception. GIG7 completed its initial public offering in August 2024. Drs. Katz and Dinu also co-founded GigInternational1, a Private-to-Public Equity (PPE) company formed for the purpose of acquiring a company in the TMT, aerospace and defense, mobility and semiconductor industries with a particular emphasis on the EMEA market. GigInternational1 completed its initial public offering in May 2021, and Dr. Dinu served as a director beginning with the inception of GigInternational1 and as the Chief Executive Officer, President and Secretary of GigInternational1 beginning in March 2021. In November 2022, GigInternational1 decided to liquidate and dissolve the company rather than pursue a business combination, and in December 2022, GigInternational1 delisted from Nasdaq after liquidating its trust account. Dr. Dinu also holds a 50% membership interest in GigManagement, LLC, and has served as a managing member of GigManagement, LLC since its inception. From April 2017 to May 2019, Dr. Dinu was the Vice President and General Manager of IDT’s Optical Interconnects Division. Prior to that, she held several executive-level positions at GigPeak, including Executive Vice President and Chief Operation Officer from April 2016 until it was acquired by IDT in April 2017, and before that, as its Executive Vice President of Global Sales and Marketing from August 2015 to April 2016, and as its Senior Vice President of Global Sales and Marketing from December 2014 to August 2015. From February 2014 to September 2017, Dr. Dinu was a member of the board of directors of Brazil-Photonics, in Campinas, Brazil, a joint venture that GigPeak established with the Centro de Pesquisa e Desenvolvimento em Telecomunicações (CPqD). From 2001 to 2008, Dr. Dinu was Vice President of Engineering at Lumera (Nasdaq: LMRA). Lumera was acquired by GigPeak in 2008, and Dr. Dinu joined GigPeak at that time. Dr. Dinu holds a B.Sc. in Physics and Ph.D. in Solid State Condensed Matter
113
Physics from the University of Bucharest, and an Executive-M.B.A. from Stanford University. She also has a Corporate Director certificate from Harvard Business School, after completing the certification for Audit Committees and Compensation Committees in 2021 and Making Corporate Boards More Effective in 2022. Dr. Dinu is married to Dr. Katz, our Chairman of the Board.
We believe Dr. Dinu is qualified to serve on the Board based on her business experience as a board member of a publicly-listed company and her investing experience.
Anastas Budagov, Chief Financial Officer.
Mr. Budagov has served as the Chief Financial Officer of QT Imaging since December 2023. Mr. Budagov served as a consultant at CBIZ APG, through which provided consulting services to public and private clients from 2022 until he became our Chief Financial Officer. Mr. Budagov previously provided financial consulting services to private and public companies while at Acilon Consulting LLC, a boutique accounting firm, from 2017 until 2022, serving as acting revenue director at Natera, Inc., a public biotech company, acting finance director at Kodiak Sciences, Inc., a public life science company, and a senior manager of more than five IPO projects for clients in the medical device, life science, and biotech industries. Immediately prior, Mr. Budagov worked at The Siegfried Group, where he was a contractor at Ernst and Young from 2013 to 2016, and advisor to management teams of public companies regarding audit processes, internal controls, and commercial contracts in 2017. Mr. Budagov also has four years of accounting experience, having served as senior accountant at regional public accounting firms. He earned his Bachelor of Science degree in accounting from George Mason University in Fairfax, VA and has been a Certified Public Accountant since 2013 in the State of Virginia.
We believe that Mr. Budagov is qualified to serve in the capacity of the Company’s Chief Financial Officer based on his 15 years of accounting and consulting experience.
Directors
Dr. Avi S. Katz co-founded GigCapital5 together with Dr. Dinu and has served as the Chairman of the Board since the inception of GigCapital5 in January 2021. Dr. Katz had also been GigCapital5’s Chief Executive Officer and President for a short period of time before Dr. Dinu substituted for him as GigCapital5’s Chief Executive Officer and President. Dr. Katz is the sole manager of GigAcquisitions5, which was our founding stockholder, and through it holds an interest in our securities held by GigAcquisitions5. Dr. Katz also holds a 50% membership interest in GigManagement, LLC, and has served as a managing member of GigManagement, LLC since its inception. Dr. Katz has spent approximately 35 years in international executive positions within the TMT industry working for privately held start-ups, and publicly traded middle-cap companies and large enterprises. After the sale of GigPeak (also known as GigOptix, NYSE GIG), which he founded and bootstrapped in April 2007 to IDT International (NYSE IDT) in April 2017, in October 2017, Dr. Katz founded GigCapital Global as a serial issuer of private-to-public equity (PPE) entities, also known as special-purpose-acquisition-company (SPAC). Dr. Katz co-founded GIG7 with Dr. Dinu, and Dr. Katz has served as the Chief Executive Officer and the Chairman of the board since the inception of GIG7 in May 2024. GIG7 completed its initial public offering in August 2024, raising $200 million. It is listed on Nasdaq and trades under the ticker symbol “GIG.” In September 2017 he founded GigCapital, Inc. (“GIG1”), company formed for the purpose of acquiring a company in the TMT industry. GIG1 completed its initial public offering in December 2017, in which it sold 14,375,000 units at price of $10.00 per unit, with each unit consisting of one share of GIG1 common stock, three-fourths (3/4) of one warrant to purchase one share of GIG1 common stock and one right to receive one-tenth (1/10) of one share of GIG1 common stock, generating aggregate proceeds of approximately $144 million. On February 22, 2019, GIG1 entered into a stock purchase agreement to acquire Kaleyra S.p.A. at about transaction enterprise value of $187 million with combined cash and/or promissory note consideration of $15 million. The transaction closed on November 25, 2019, and GIG1 was renamed Kaleyra, Inc. and listed on the NYSE American stock exchange under the symbol “KLR” (and since that time, Kaleyra uplisted to the NYSE). In November 2023, KLR was sold to Tata Communications at a transaction enterprise value of about $320 million in a cash deal and ceased to exist as a public company. Dr. Katz served as the Chairman of the board and Secretary of Kaleyra since the consummation of the transaction in November 2019 until the acquisition by Tata. In this capacity, Dr. Katz steered many restructurings and refinancings, including the acquisition of mGage from Blackstone for about $225 million in a cash and stock deal in June 2021. Prior to that time, Dr. Katz served as the Executive Chairman, Secretary, and Chief Executive Officer of GIG1. In March 2019, Dr. Katz founded GIG2, a Private-to-Public Equity (PPE) company formed for the purpose of acquiring a company in the TMT industry. GIG2 completed its initial public offering in June 2019, in which it sold 17,250,000 units at a per unit price of $10.00, with each unit consisting of one share of GIG2 common stock, one warrant to purchase one share of GIG2 common stock, and one right to receive one-twentieth (1/20) of one share of GIG2 common stock, generating aggregate proceeds of about $173 million. On June 8, 2021, GIG2 completed its business combination with each of UpHealth Holdings, Inc. and Cloudbreak Health, LLC, and the Company changed its name to
114
UpHealth, Inc. and was listed on the NYSE under the new ticker symbol “UPH”, where it remained listed until 2024 when it was delisted from the NYSE and commenced trading on the OTC Pink under the new ticker symbol “UPHL.” Dr. Katz initially served as the Chief Executive Officer of GIG2 until August 2019, when Dr. Dinu substituted for him in that position. He also served as the Executive Chairman and Secretary of GIG2 since inception until the closing of the business combination in June 2021, when Dr. Katz was appointed as the Co-Chairman of the board of directors of UpHealth, becoming the sole Chairman of the board of UpHealth in June 2022. In this capacity, Dr. Katz was steering many restructurings and refinancings of the company, including the sales of two divisions of the company, to IGI for $56 million in a cash deal in June 2023 and the sale of Cloudbreak for $180 million in a cash deal to GTCR in March 2024. In February 2020, Drs. Katz and Dinu co-founded GIG3, a Private-to-Public Equity (PPE) company formed for the purpose of acquiring a company in the TMT industry. GIG3 completed its initial public offering in May 2020, in which it sold 20,000,000 units at a per unit price of $10.00, with each unit consisting of one share of GIG3 common stock and three-fourths (3/4) of one warrant to purchase one share of GIG3 common stock, generating aggregate proceeds of $200 million. On May 6, 2021, GIG3 completed its business combination with Lightning Systems, Inc., which does business as Lightning eMotors, and the Company retained such name. Lightning eMotors, Inc. was listed on the NYSE under the new ticker symbol “ZEV,” but now is listed on the OTC Expert Market under the ticker symbol “ZEVY.” Dr. Katz served as the Chief Executive Officer, Executive Chairman and Secretary of GIG3 since its inception until the closing of the business combination in May 2021, when Dr. Katz was appointed as the Co-Chairman of the board of directors of Lightning eMotors and served in that position until October 2021 when he did not stand for reelection to the board of directors. In December 2020, Drs. Katz and Dinu co-founded GIG4, a Private-to-Public Equity (PPE) company formed for the purpose of acquiring a company in the TMT and sustainable industries. GIG4 completed its initial public offering in February 2021, in which it sold 35,880,000 units at a per unit price of $10.00, with each unit consisting of one share of GIG4 common stock and one-third (1/3) of one (1) warrant to purchase one share of GIG4 common stock, generating aggregate proceeds of about $359 million. GIG4 listed on Nasdaq under the symbol “GIG.” In June 2021, GIG4 announced its agreement for a business combination with BigBear.ai Holdings, LLC. The business combination between GIG4 and BigBear.ai Holdings, LLC closed in December 9, 2021, and GIG4 was renamed BigBear.ai Holdings, Inc. BigBear.ai moved its listing from Nasdaq to the NYSE, where it is listed under the ticker symbol “BBAI.” Dr. Katz served as the Executive Chairman of GIG4 from its inception until the closing of the business combination with BigBear.ai on December 9, 2021, and since then and until March 2024, has continued to serve as a member of the board of directors of BigBear.ai. In February 2021, Drs. Katz and Dinu co-founded GigInternational1, Inc. a Private-to-Public Equity (PPE) company formed for the purpose of acquiring a company in the TMT, aerospace and defense, mobility and semiconductor industries with a particular emphasis on the EMEA market. GigInternational1 completed its initial public offering in May 2021, in which it sold 20,900,000 units at a per unit price of $10.00, with each unit consisting of one share of GigInternational1 common stock and one-half (1/2) of one (1) warrant to purchase one share of GigInternational1 common stock, generating aggregate proceeds of $209 million. GigInternational1 listed on Nasdaq under the symbol “GIW,” but in November 2022, decided to liquidate and dissolve the company rather than pursue a business combination, and in December 2022, GigInternational1 delisted from Nasdaq after liquidating its trust account. Dr. Katz was the Executive Chairman of GigInternational1 since its inception. Prior to launching his first Private-to-Public (PPE) company in 2017, Dr. Katz dedicated 10 years to incept and bootstrap, develop and manage GigPeak (NYSE American: formerly GIG), originally known as GigOptix, Inc. He served as Chairman of the Board, Chief Executive Officer and President of GigOptix / GigPeak from its inception in 2007 until its sale in April 2017 to IDT International (Nasdaq: IDTI) for $250 million in cash. While Dr. Katz was at GigPeak’s helm, the company completed 10 M&A deals. From 2003 to 2005, Dr. Katz was the chief executive officer, president, and member of the board of directors of Intransa, Inc. From 2000 to 2003, Dr. Katz was the chief executive officer, president and a member of the board of directors of Equator Technologies. Prior to it, Dr. Katz held several leadership positions over the span of his career within the TMT industry since serving as member of Technical Staff at AT&T Bell Laboratories between 1988 and 1994, and made numerous angel investments in high-tech companies around the world, being a serial entrepreneur. He holds many U.S. and international patents, authored and co-authored more than 350 published scientific and technical articles in reputable journals, and is the editor of a number of technical books. Dr. Katz is a global philanthropist, and among many other activities, serves as board member of the NY Philharmonic Company. He is a graduate of the 1976 class of the Israeli Naval Academy, graduate of the 1979 USA Naval ASW class, and holds a B.Sc. and Ph.D. in Materials from the Technion (Israel Institute of Technology). Dr. Katz is married to Dr. Dinu, our Chief Executive Officer and one of our directors.
We believe that Dr. Katz is qualified to serve as Chairman of the Board based on his business experience as a founder, inventor, chief executive officer and director of a publicly-listed company and his investing experience.
Dr. John C. Klock served as the Chief Executive Officer of QT Imaging, Inc. from 2014 to 2024, and as a Director and Founder of QT Imaging, Inc. and its predecessors since 2011. Following the Closing of the Business Combination in March 2024, he has served as a member of our Board, and briefly served as our Chief Executive Officer until Dr. Dinu
115
took over that role shortly after the closing of the Business Combination. Prior to serving in these positions with QT Imaging, Dr. Klock was involved in the start-up of five medical companies, including as Co-Founder and President of BioMarin Pharmaceutical, Inc., which successfully commercialized five FDA drugs; and Scientific Founder and Vice President of Research of Glycomed, Inc., which was acquired by Ligand Pharmaceuticals, Inc. He also personally brought to market a novel cancer treatment, the first rapid AIDS test, comprehensive tests for detecting metabolic diseases in children, and several drugs for treating pediatric genetic conditions. Dr. Klock has authored over 70 peer-reviewed medical and scientific publications and has been granted eight patents.
We believe Dr. Klock is qualified to serve on our Board due to his intimate knowledge of the business and operations of QT Imaging, including the scientific basis, regulatory requirements, sales and marketing channels of QT Imaging’s products, as well as Dr. Klock’s extensive medical experience.
Mr. Ross Taylor joined our board in March 2024. Ross Taylor is the Chief Financial Officer of BillionToOne, Inc. Mr. Taylor served as Senior Vice President and Chief Financial Officer of Codexis, Inc. from August 2019 to January 2023. Previously, Mr. Taylor served as Chief Financial Officer, Vice President of Finance and Secretary of Abaxis, Inc. from August 2015 through July 2018 at which time Zoetis acquired Abaxis, Inc. Also, Mr. Taylor served as Vice President of Business Development & Investor Relations at Abaxis, Inc. from October 2014 through July 2015. Prior to Abaxis, Mr. Taylor worked in equity research for various Wall Street firms including CL King & Associates, where he was Senior Vice President/Equity Research Analyst from July 2005 through October 2014, UBS, and Smith Barney. Mr. Taylor earned a Master of Business Administration degree at Columbia Business School and a Bachelor’s degree in Economics from Duke University.
We believe that Mr. Taylor is qualified to serve on the Board based on his business experience and his financial expertise.
Mr. Daniel Dickson joined the QT Imaging, Inc. Board in November 2022, and has continued to serve on our Board following the closing of the Business Combination in March 2024. Mr. Dickson began his executive management career in 1980, when he joined General Electric Company. From 1980 until 1987, he held a number of strategic and operational roles and had responsibility for a $300 million business in the company’s consumer electronics division. In 1987, Mr. Dickson left GE to join a startup company that brought advanced technology to consumer products retailing. As SVP Marketing, he helped grow revenue to $12 million and was a key player in the company’s IPO in 1989. In 1990, Mr. Dickson moved to California, where he became President and COO of a privately held data management company located in Santa Monica, CA. After repositioning the company to take advantage of the growing trend toward personalized marketing and internet-based market research, he was brought to San Francisco by the venture capital firm Draper Fisher Jurvetson in 1996 to serve as President and CEO of one of their early-stage internet companies. Based on this experience, he joined The Brenner Group, Inc., in 1998 where he built that company’s interim CEO practice. During that period, he also served as a “parachute” CEO and was retained by multiple San Francisco Bay Area venture firms to manage and reposition their portfolio companies, including Armus Corporation, a data management firm that focused on medical outcomes (acquired by Health Catalyst Capital Management in 2022), and Vital Transport, a start-up company involved in organ transport. In 2003, Mr. Dickson returned to the East Coast where he became President and CEO of Best Cellars, Inc., an innovative wine retailer with operations in five states. After doubling sales and creating a significant internet business, he negotiated the company’s acquisition by the $9 billion publicly held Great Atlantic & Pacific Grocery chain in 2007. After the acquisition, Mr. Dickson was retained as a “virtual COO” for the company’s $200 million wine, beer, and spirits operation, where he remained until 2011. From 2011 to 2018, he served as a board member and later advisor to the board of The Winebow Group, an $800 million fine wine distributor with locations in 19 states across the country. From 2018 until 2021 he acted as CFO of the Latin American Auto Group, an initiative led by automotive industry pioneer Marshall S. Cogan. He currently maintains an independent consulting practice focusing on executive coaching and strategic analysis, and is an executive coach affiliated with SUMMi7 LLC in Dallas, TX. Mr. Dickson holds an M.B.A., with Distinction, from Harvard’s Graduate School of Business Administration (1980), and a B.S. in Public Communication, Summa Cum Laude, from Boston University (1974), and is a registered Agile Product Owner and Scrum Master.
We believe Mr. Dickson is qualified to serve on our Board because of his more than 30 years of C-level experience and expertise working in companies ranging from startups to Fortune 50 and his experience in industries from consumer products to enterprise software, as well as his proven ability to focus and scale a company.
Mr. James Greene joined our Board in March 2024. Mr. Greene serves as a director of Umpqua Bank (Nasdaq: UMPQ) and Uphealth, Inc. (OTC Pink: UPHL). He is Founder and Managing Partner of Sky D Ventures, LLC, a private equity and advisory services company serving the financial services and FinTech global market. Prior to Sky D Ventures, Mr. Greene was a general partner with an incubator of start-ups focused on digital platforms and solutions from November 2013 to
116
October 2015. He was previously a Vice President with Cisco Systems, Inc. (Nasdaq: CSCO) in its Global Advanced Services Organization, a position he held from February 2012 to September 2013. He joined Cisco in 2005 as Vice President and Global Head of its Financial Services Consulting Business. From there he served as leader of Cisco’s global Strategic Partner Organization. Before Mr. Greene’s tenure at Cisco and Accenture, he generated significant growth as president and CEO of Abilizer, a portal technology start-up company, as managing director at Capgemini, and as global head of financial services at TeleTech.
We believe that Mr. Greene is qualified to serve on our Board based on his leadership experience with technology companies, as well as his business development and finance experience.
Professor Zeev Weiner joined our Board upon the closing of the Business Combination in March 2024. Professor Weiner has been the director of the Department of Obstetrics and Oncology at the Rambam Health Care Campus in Haifa, Israel since 2014. He is currently the president of the OB/GYN Society of Northern Israel, a member of Israel’s National OB/GYN Committee, a member of the Obstetrics and Gynecology Teaching Committee at Technion – Israel Institute of Technology’s Rappaport Faculty of Medicine, a member of Life journal’s editorial board, an organizer of post-graduate courses in obstetrics for resident physicians in northern Israel and a reviewer of the publication Prenatal Diagnosis. Professor Weiner also sits on the Rappaport Faculty of Medicine at Technion – Israel Institute of Technology in Haifa, Israel, a leading global medical school, as both a clinical professor and an associate clinical professor, positions Professor Weiner has held since 2022 and 2007, respectively. Since 1987, Professor Weiner has served as an instructor of obstetrics and gynecology to clinical medical students and a lecturer of obstetrics and gynecology to fifth year obstetrics and gynecology residents, in each case through the Rappaport Faculty of Medicine at Technion – Israel Institute of Technology. Since 2002, Professor Weiner has also served as lecturer of an ultrasound and doppler in obstetrics and gynecology course at the Israel School of Ultrasound in Obstetrics and Gynecology. Previously, Professor Weiner was the director Ultrasound in Obstetrics and Gynecology Rambam Health Care Campus from 2005 to 2014, the director of Maternal Fetal Medicine in the Department of Obstetrics and Gynecology at the Lutheran Medical Center in Brooklyn, NY from 2003 to 2005, and the director of Perinatology at the Emek Medical Center in Afula, Israel from 1998 to 2003. In addition, Professor Weiner served as head of the OB/GYN Exam Preparation Committee at Technion – Israel Institute of Technology’s Rappaport Faculty of Medicine from 2009 to 2012. In connection with these academic activities, Professor Weiner’s research has been published numerous times in various medical and related academic journals. Professor Weiner received his MD from Tel Aviv University’s Sackler Faculty of Medicine in 1986, and an MHA from Tel Aviv University’s Sackler Faculty of Medicine in 2012. Professor Weiner received the “Outstanding Sixth Year Student” in 1986 in honor of his high academic achievement as a medical student. Further, in 2005, Professor Weiner received the National Faculty Award in the field of Obstetrics and Gynecology from the American College of Obstetrics and Gynecology’s Council on Resident Education in Obstetrics and Gynecology and the APGO Excellence in Teaching Award from the Association of Professors of Gynecology and Obstetrics at Lutheran Medical Center’s Department of Obstetrics and Gynecology.
We believe that Professor Weiner is qualified to serve on our Board based on his business experience and his obstetrics and oncology expertise.
Role of Board in Risk Oversight
The Board has an active role, as a whole and also at the committee level, in overseeing the management of the Company’s risks. The Board is responsible for general oversight of risks and regular review of information regarding the Company’s risks, including credit risks, liquidity risks, and operational risks. The Compensation Committee is responsible for overseeing the management of risks relating to the Company’s executive compensation plans and arrangements. The Audit Committee is responsible for overseeing the management of risks relating to accounting matters and financial reporting and potential conflicts of interest. The Nominating and Corporate Governance Committee is responsible for overseeing the management of risks associated with the independence of the Board. Although each committee is responsible for evaluating certain risks and overseeing the management of such risks, the entire Board is regularly informed through discussions from committee members about such risks.
Board Composition and Classification
The Board consists of seven members. In accordance with the Charter, the Board is classified. The Board believes it is in the best interests of the Company for the Board to be classified into three classes, each comprising as nearly as possible one-third of the directors to serve three-year terms, and only one class of directors will be elected at each annual meeting of
117
stockholders, with the other classes continuing for the remainder of their respective three-year terms. The Board consists of the following members:
•the Class I directors are Daniel Dickson and James Greene and their terms will expire at the annual meeting of stockholders to be held in 2025;
•the Class II directors are Ross Taylor and Professor Zeev Weiner and their terms will expire at the annual meeting of stockholders to be held in 2026; and
•the Class III directors are Dr. Avi S. Katz, Dr. Raluca Dinu and Dr. John Klock and their terms will expire at the annual meeting of stockholders to be held in 2027.
At each annual meeting of stockholders, upon the expiration of the term of a class of directors, the successor to each such director in the class will be elected to serve from the time of election and qualification until the third annual meeting following their election and until their successor is duly elected and qualified, in accordance with the Charter. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the Company’s directors.
This classification of the Company’s directors may have the effect of delaying or preventing changes in control of the Company.
Board Committees
The standing committees of the Board consist of the Audit Committee, the Compensation Committee and a Nominating and Corporate Governance Committee, each of which has the composition and the responsibilities described below. Additionally, from time to time, special committees may be established under the direction of the Board when the Board deems it necessary or advisable to address specific matters.
The Chief Executive Officer and other executive officers regularly report to the non-executive directors and each standing committee to ensure effective and efficient oversight of its activities and to assist in proper risk management and the ongoing evaluation of management controls.
Audit Committee
The members of the Company’s Audit Committee are Ross Taylor, Professor Zeev Weiner and James Greene. Mr. Taylor is the Chair of the Audit Committee and the “audit committee financial expert,” as that term is defined under the SEC rules implementing Section 407 of SOX, and possesses financial sophistication, as defined under the rules of Nasdaq. The Company’s Audit Committee oversees the Company’s corporate accounting and financial reporting process and assists the Board in monitoring the Company’s financial systems. The Company’s Audit Committee also:
•assists the Board in the oversight of (1) the accounting and financial reporting processes of the Company and the audits of the consolidated financial statements of the Company, (2) the preparation and integrity of the consolidated financial statements of the Company, (3) the compliance by the Company with financial statement and regulatory requirements, (4) the performance of the Company’s internal finance and accounting personnel and its independent registered public accounting firm, and (5) the qualifications and independence of the Company’s independent registered public accounting firm;
•reviews with each of the internal auditors and independent registered public accounting firm the overall scope and plans for audits, including authority and organizational reporting lines and adequacy of staffing and compensation.
•reviews and discusses with management and internal auditors the Company’s system of internal control and discussing with the independent registered public accounting firm any significant matters regarding internal controls over financial reporting that have come to its attention during the conduct of its audit;
•reviews and discusses with management, internal auditors and the independent registered public accounting firm the Company’s financial and critical accounting practices, and policies relating to risk assessment and management;
•receives and reviews reports of the independent registered public accounting firm discussing (1) all critical accounting policies and practices to be used in the independent registered public accounting firm’s audit of the Company’s consolidated financial statements, (2) all alternative treatments of financial information within GAAP
118
that have been discussed with management, ramifications of the use of such alternative disclosures and treatments, and the treatment preferred by the independent registered public accounting firm, and (3) other material written communications between the independent registered public accounting firm and management, such as any management letter or schedule of unadjusted differences;
•reviews and discusses with management and the independent registered public accounting firm the annual and quarterly consolidated financial statements and section entitled “Management’s Discussion and Analysis of Financial Conditions and Results of Operations” of the Company prior to the filing of the Company’s annual report on Form 10-K and quarterly reports on Form 10-Q;
•reviews, or establishes, standards for the type of information and the type of presentation of such information to be included in, earnings press releases and earnings guidance provided to analysts and rating agencies;
•discusses with management and the independent registered public accounting firm any changes in the Company’s critical accounting principles and the effects of alternative GAAP methods, off-balance sheet structures and regulatory and accounting initiatives;
•reviews material pending legal proceedings involving the Company and other contingent liabilities;
•meets periodically with the Chief Executive Officer, Chief Financial Officer, the senior internal auditing executive and the independent registered public accounting firm in separate executive sessions to discuss results of examinations;
•reviews and approves all transactions between the Company and related parties or affiliates of the officers of the Company requiring disclosure under Item 404 of Regulation S-K prior to the Company entering into such transactions;
•establishes procedures for the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters, and the confidential, anonymous submissions by employees or contractors of concerns regarding questionable accounting or accounting matters;
•reviews periodically with the Company’s management, independent registered public accounting firm and outside legal counsel (i) legal and regulatory matters which may have a material effect on the consolidated financial statements, and (ii) corporate compliance policies or codes of conduct, including any correspondence with regulators or government agencies and any employee complaints or published reports that raise material issues regarding the Company’s consolidated financial statements or accounting policies and any significant changes in accounting standards or rules promulgated by the Financial Accounting Standards Board, the SEC or other regulatory authorities; and
•establishes policies for the hiring of employees and former employees of the independent registered public accounting firm.
The Company’s audit committee operates under a written charter, which satisfies the applicable rules of the SEC and the listing standards of Nasdaq.
Compensation Committee
The Company has established a Compensation Committee of the Board. The members of our Compensation Committee are Daniel Dickson, Professor Zeev Weiner, Ross Taylor and James Greene. Mr. Greene serves as Chair of the Compensation Committee. The Company has adopted a Compensation Committee charter, which details the purpose and responsibility of the compensation committee, including:
•reviewing the performance of the Chief Executive Officer and executive management;
•assisting the Board in developing and evaluating potential candidates for executive positions (including the Chief Executive Officer);
•reviewing and approving goals and objectives relevant to the Chief Executive Officer and other executive officer compensation, evaluate the Chief Executive Officer’s and other executive officers’ performance in light of these corporate goals and objectives, and set Chief Executive Officer and other executive officer compensation levels consistent with its evaluation and the Company philosophy;
119
•approving the salaries, bonus and other compensation for all executive officers;
•reviewing and approving compensation packages for new corporate officers and termination packages for corporate officers as requested by management;
•reviewing and discussing with the Board and senior officers plans for officer development and corporate succession plans for the Chief Executive Officer and other senior officers;
•reviewing and making recommendations concerning executive compensation policies and plans;
•reviewing and recommending to the Board the adoption of or changes to the compensation of the Company’s directors;
•reviewing and approving the awards made under any executive officer bonus plan, and provide an appropriate report to the Board;
•reviewing and making recommendations concerning long-term incentive compensation plans, including the use of stock options and other equity-based plans, and, except as otherwise delegated by the Board, acting on as the “Plan Administrator” for equity-based and employee benefit plans;
•approving all special perquisites, special cash payments and other special compensation and benefit arrangements for the Company’s executive officers and employees;
•reviewing periodic reports from management on matters relating to the Company’s personnel appointments and practices;
•assisting management in complying with the Company’s proxy statement and annual report disclosure requirements;
•issuing an annual report of the compensation committee on executive compensation for the Company’s annual proxy statement in compliance with applicable SEC rules and regulations;
•annually evaluating the committee’s performance and the committee’s charter and recommending to the Board any proposed changes to the charter or the committee; and
•undertaking all further actions and discharge all further responsibilities imposed upon the committee from time to time by the Board, the federal securities laws or the rules and regulations of the SEC.
The charter also provides that the Compensation Committee may, in its sole discretion, retain or obtain the advice of a compensation consultant, independent legal counsel or other adviser and will be directly responsible for the appointment, compensation and oversight of the work of any such adviser. However, before engaging or receiving advice from a compensation consultant, external legal counsel or any other adviser, the Compensation Committee will consider the independence of each such adviser, including the factors required by Nasdaq and the SEC.
Nominating and Corporate Governance Committee
The members of the Company’s Nominating and Corporate Governance Committee are Ross Taylor, James Greene, Daniel Dickson and Professor Zeev Weiner. Professor Zeev Weiner is the Chair of the Company’s Nominating and Corporate Governance Committee. The Company’s Nominating and Corporate Governance Committee oversees and assists the Board in reviewing and recommending nominees for election as directors. Specifically, the Nominating and Corporate Governance Committee will:
•develop and recommend to the Board the criteria for appointment as a director;
•identify, consider, recruit and recommend candidates to fill new positions on the Board;
•review candidates recommended by stockholders;
•conduct the appropriate and necessary inquiries into the backgrounds and qualifications of possible candidates; and
•recommend director nominees for approval by the Board and election by the stockholders at the next annual meeting.
120
The Company’s Nominating and Corporate Governance Committee operate under a written charter which satisfies the applicable rules of the SEC and the listing standards of Nasdaq.
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics applicable to the Company’s directors, officers, and employees, including our principal executive officer, principal financial officer, principal accounting officer or controller or, persons performing similar functions, in accordance with applicable federal securities laws. We have filed a copy of our form of Code of Business Conduct and Ethics and our board committee charters as exhibits to this annual report . You are able to review these documents by accessing our public filings at the SEC’s web site at www.sec.gov. The Company’s Code of Business Conduct and Ethics is also available on the investor relations section of our website at www.qtimaging.com. We intend to disclose any amendments to or waivers of our Code of Business Conduct and Ethics in a Current Report on Form 8-K on our website identified above. Information contained on our website is not incorporated by reference into this annual report and should not be considered to be part of this annual report.
Compensation Committee Interlocks and Insider Participation
None of the members of our Compensation Committee is or has been an officer or employee of the Company. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the board of directors, or compensation committee (or other board committee performing equivalent functions) of any entity that has one or more executive officers serving on the Board or Compensation Committee.
Limitation on Liability and Indemnification of Directors and Officers
The Charter provides that our officers and directors will be indemnified by us to the fullest extent authorized by Delaware law, as it now exists or may in the future be amended. In addition, the Charter provides that our directors will not be personally liable for monetary damages to us or our stockholders for breaches of their fiduciary duty as directors, unless they violated their duty of loyalty to us or our stockholders, acted in bad faith, knowingly or intentionally violated the law, authorized unlawful payments of dividends, unlawful stock purchases or unlawful redemptions, or derived an improper personal benefit from their actions as directors.
We have entered into agreements with our officers and directors to provide contractual indemnification in addition to the indemnification provided for in our Charter. Our Bylaws also permit us to secure insurance on behalf of any officer, director or employee for any liability arising out of his or her actions, regardless of whether Delaware law would permit such indemnification. We purchase a policy of directors’ and officers’ liability insurance that insures our officers and directors against the cost of defense, settlement or payment of a judgment in some circumstances and insures us against our obligations to indemnify our officers and directors.
These provisions may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against officers and directors, even though such an action, if successful, might otherwise benefit us and our stockholders. Furthermore, a stockholder’s investment may be adversely affected to the extent we pay the costs of settlement and damage awards against officers and directors pursuant to these indemnification provisions.
We believe that these provisions, the directors’ and officers’ liability insurance and the indemnity agreements are necessary to attract and retain talented and experienced officers and directors.
Insider Trading Policy
We have adopted an insider trading policy governing the purchase, sale and/or other dispositions of its securities by its directors, officers and employees that we believe is reasonably designed to promote compliance with insider trading laws, rules and regulations and the Nasdaq listing standards. The foregoing summary of our insider trading policy does not purport to be complete and is qualified in its entirety by reference to the full text thereof attached hereto as Exhibit 19.1.
Item 11: Executive Compensation
Compensation Discussion and Analysis
The following discussion and analysis of our executive compensation philosophy, objectives and design, our compensation-setting process, the components of our executive compensation program, and the decisions made for compensation in respect of 2024 for our executive officers should be read together with the compensation tables and related disclosures set forth below. The discussion in this section contains forward-looking statements that are based on our current
121
considerations and expectations relating to our executive compensation programs and philosophy. As our business and our needs evolve, the actual amount and form of compensation and the compensation programs that we adopt may differ materially from current or planned programs as summarized in this section.
Overview
This section provides an overview of our executive compensation program, including a narrative description of the material factors necessary to understand the information disclosed in the summary compensation table below.
In evaluating our overall executive compensation program and decisions for the 2024 fiscal year, including awards under our compensation programs, the Compensation Committee considered a number of factors, including that 2024 was our first year operating as a public company, incentivizing the achievement of both strategic enterprise and financial objectives and our position as a transformational company. Going forward, the Compensation Committee will be making any determinations as it relates to the payout of the previous year’s compensation programs as well as the adoption of any performance measures for the current fiscal year. This allows the Compensation Committee to have a good understanding of the prior fiscal year financial performance in order to evaluate the performance of our named executive officers (each, a “NEO”) against previously adopted performance measures as well as develop plans and performance metrics based on the annual operating plan for the current fiscal year.
For the year ended December 31, 2024, our NEOs were:
| Name | Age | Position | ||||||||||||
Dr. Raluca Dinu(1) | 51 | Chief Executive Officer and Director (Class III) | ||||||||||||
Dr. John C. Klock(2) | 80 | Former Chief Executive Officer and Director (Class III) | ||||||||||||
Anastas Budagov(3) | 37 | Chief Financial Officer | ||||||||||||
______________
(1)Dr. Raluca Dinu has served as our Chief Executive Officer and Class III Director since March 12, 2024, when she was appointed by the Board to replace Dr. John C. Klock, effective immediately.
(2)Dr. John C. Klock served as our Chief Executive Officer since our inception and until March 12, 2024, when he was replaced by the Board with Dr. Dinu.
(3)Mr. Budagov has served as our Chief Financial Officer since December 2023, and the Board ratified his prior appointment on March 12, 2024.
Compensation Philosophy and Objectives
The Company has developed an executive compensation program that is consistent with the compensation policies and philosophies of the Compensation Committee and the Board, which are designed to align compensation with the Company’s business objectives and the creation of stockholder value, while enabling the Company to attract, motivate and retain individuals who contribute to its long-term success. Decisions on the executive compensation program are made by the Compensation Committee.
The objectives of the Company’s executive compensation program are to encourage retention and recruitment of high-performing executives, to motivate employees and align executive interests across the organization and with the Company’s stockholders, to reward sustainable financial performance, accountability and innovation, to create consistence with the Company’s strategy and culture (mission, vision and values) and to balance innovation and performance with risk. In setting executive compensation in 2024, the Compensation Committee took into account the Company’s strategy, culture and stage in defining plan feature tradeoffs. The Compensation Committee also looked to manage exceptions to its approach based upon the individual profiles of various members of the Company’s management.
Decisions regarding executive compensation reflect a belief that the executive compensation program must be competitive in order to attract and retain highly competent executive officers as well as include a significant element of “pay for performance.” Total compensation will be comprised of base salary, short-term incentive and long-term incentive. A significant portion of compensation for the members of management of the Company is tied to annual performance objectives. All elements of the compensation are defined in absolute dollar values. Further, the Compensation Committee seeks to tie our executive compensation levels to the compensation practices of our peer companies.
122
Base Salary
Base salaries for our NEOs are established based on the individual’s scope of responsibilities, experience and market factors. The base salary is an annual total cash salary paid over 12 months in equal amounts. The Compensation Committee typically reviews base salaries on an annual basis, referencing peer group and survey data to understand the marketplace for individuals in similar positions. No formulaic base salary increases are provided to our NEOs; however, annual merit increases are provided when the Compensation Committee determines that such increases are warranted in light of national salary increase levels, salary levels within companies in our peer group, individual performance and/or overall Company performance. We pay base salaries to attract, recruit and retain qualified employees. The base salaries for 2024 for our NEOs take into account the initial base amount set forth in the executive’s respective employment agreement or employment offer letter, as applicable, and the scope of the executive’s responsibilities, individual contributions, prior experience and sustained performance.
| Name | 2024 Base Salary | |||||||
Dr. Raluca Dinu(1) | $ | 470,000 | ||||||
Dr. John C. Klock(2) | $ | — | ||||||
Anastas Budagov(3) | $ | 380,000 | ||||||
________________
(1)Dr. Raluca Dinu’s base salary is based on her employment agreement dated March 12, 2024.
(2)Dr. John C. Klock did not receive a base salary.
(3)Mr. Budagov’s base salary is based on his employment agreement dated March 12, 2024.
Annual Bonuses
The Company uses annual cash incentive bonuses for the NEOs to tie a portion of their compensation to financial and operational objectives achievable within the applicable fiscal year. The annual cash incentive bonus is expressed as a percentage of an individual’s base salary. The Compensation Committee set the performance targets using two financial metrics for the 2024 fiscal year: total revenue; and cash balance. Determination of the achievement of the targets is based upon the full year financial results following the completion of the audit of the Company’s consolidated financial statements. Following the end of the year, the Compensation Committee will determine the extent to which the performance targets were achieved and the amount of the award that will payable to the NEOs.
Equity-Based Awards
The Company uses equity-based awards to reward long-term performance of the NEOs. The Company believes that providing a meaningful portion of the total compensation package in the form of equity-based awards aligns the incentives of its NEOs with the interests of its stockholders and serves to motivate and retain the individual NEOs. Any awards would be made in accordance with the executive compensation program discussed above. Although the Company has not used a specific predetermined schedule to grant equity-based awards as 2024 was the first year that the Company made such awards as a public company, it is the policy of the Company regarding our grants of equity-based awards that the Company does not (a) backdate equity award grants, (b) time the public release of material information or (c) purposely accelerate or delay equity award grants with the intent of allowing an award recipient to benefit from a more favorable stock price. The Company is currently using time-based stock option awards to encourage long term performance.
Other Compensation
The Company maintains various employee benefit plans, including medical, dental, life insurance and 401(k) plans, in which the NEOs may participate. It also provides certain perquisites to its NEOs, subject to the Compensation Committee’s ongoing review.
Deductibility of Executive Compensation
Section 162(m) of the Code denies a federal income tax deduction for certain compensation in excess of $1.0 million per year paid to the chief executive officer, the chief financial officer, the three other most highly paid executive officers of a publicly traded corporation, and anyone previously subject to Section 162(m) as a covered employee for any taxable year beginning after December 31, 2016. It is the policy of the Company to consider the tax impact of its compensation arrangements as one factor, among others, in evaluating and determining the structure, implementation, and amount of
123
awards paid to its executive officers. However, to retain highly skilled executives and remain competitive with other employers, the Compensation Committee may authorize compensation that would not be deductible under Section 162(m) or otherwise if it determines that such compensation is in the best interests of the Company and its stockholders, and maintaining tax deductibility will not be the sole consideration taken into account in determining what compensation arrangements are in our and our stockholders’ best interests. The right to grant compensation that is not deductible is expressly reserved, and the Company may do so.
Summary of Compensation Table
The table below sets forth the annual compensation levels of our NEOs for 2024 and 2023, who as a smaller reporting company consist of the principal executive officer who serves as Chief Executive Officer of QT Imaging, and the next two most highly compensated executive officers. The compensation totals and individual amounts reflect the compensation of such officers by the Company or QT Imaging as of December 31, 2024 and 2023. In the fiscal year 2025, such totals and amounts may change based on, among other things, changes to the terms of the employment of such persons.
Name and Principal Poslition | Year | Salary ($) | Option Awards ($) (1) | Bonus ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||||||||||||
Dr. Raluca Dinu(2) Chief Executive Officer & Class III Director | 2023 | — | — | — | — | — | ||||||||||||||||||||||||||||||||
2024 | 359,731 | 279,070 | — | — | 638,801 | |||||||||||||||||||||||||||||||||
John Klock, M.D., Former President and Chief Executive Officer & Chief Medical Officer | 2023 | — | — | — | — | — | ||||||||||||||||||||||||||||||||
2024 | — | — | — | — | — | |||||||||||||||||||||||||||||||||
Anastas Budagov (3) Chief Financial Officer | 2023 | — | — | — | — | — | ||||||||||||||||||||||||||||||||
2024 | 290,846 | 153,725 | 63,333 | — | 507,904 | |||||||||||||||||||||||||||||||||
Mikel Ann Price (4) Former Chief Financial Officer | 2023 | 267,596 | — | — | 22,211 | 289,807 | ||||||||||||||||||||||||||||||||
2024 | — | — | — | — | — | |||||||||||||||||||||||||||||||||
__________________
(1)Consists of shares of common stock of the Company issuable upon vesting and exercise of stock options. The amounts in this column represent the aggregate grant date fair value computed in accordance with FASB ASC Topic 718.
(2)On March 12, 2024, the Board appointed Dr. Raluca Dinu to serve as the Chief Executive Officer of the Company, effective immediately. Dr. Raluca Dinu’s base salary per her employment agreement, which the Board approved on March 18, 2024, effective as of March 12, 2024 was $470,000. On July 3, 2024, the Board approved the grant of 550,000 stock options to Dr. Raluca Dinu. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option. One-third of the stock options granted will vest on February 15, 2025, and the remaining two-thirds of the stock options will vest quarterly over a two year period thereafter.
(3)On March 12, 2024, the Board ratified the prior appointment of Mr. Budagov as the Company’s Chief Financial Officer. Mr. Budagov’s base salary per his employment agreement, which the Board approved on March 18, 2024, effective as of March 12, 2024 was $380,000. On July 3, 2024, the Board approved the grant of 325,000 stock options to Mr. Budagov. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option. One-third of the stock options granted will vest on February 15, 2025, and the remaining two-thirds of the stock options will vest quarterly over a two year period thereafter. During 2024, Mr. Budagov received a sign-on bonus of $63,333 in accordance with his employment agreement.
(4)Effective December 8, 2023, Mikel Ann Price resigned from her full-time position as Chief Financial Officer. As part of her final termination payment, Ms. Price received a cash payment of $22,211 for earned and unused PTO.
124
Outstanding Equity Awards at Fiscal Year-End
The following table sets forth information concerning outstanding equity awards held by each of the Company’s NEOs as of December 31, 2024.
| Option Awards | ||||||||||||||||||||||||||||||||
| Name | Grant Date | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price ($) | Option Expiration Date | |||||||||||||||||||||||||||
Dr. Raluca Dinu | 07/03/2024 | — | 590,000 | $ | 0.748 | 07/03/2034 | ||||||||||||||||||||||||||
Anastas Budagov | 07/03/2024 | — | 325,000 | $ | 0.748 | 07/03/2034 | ||||||||||||||||||||||||||
Employment Arrangements with Named Executive Officers
Employment Agreement with Dr. Raluca Dinu
On March 12, 2024, the Board appointed Dr. Raluca Dinu, who is also a member of the Board, to be employed as its Acting Chief Executive Officer effective as of March 12, 2024. Dr. Dinu will report to the Board. On March 18, 2024, the Board approved an employment agreement (the “CEO Employment Agreement”) between Dr. Dinu and the Company, effective as of March 12, 2024, governing the terms of Dr. Dinu’s employment by the Company, which the Company and Dr. Dinu then entered into.
Under the terms of the CEO Employment Agreement, Dr. Dinu will be hired on an “at will” basis and shall serve as the Company’s Chief Executive Officer on an interim but full-time basis, performing her duties and responsibilities in such capacity. The CEO Employment Agreement provides that Dr. Dinu will serve as the Company’s Chief Executive Officer until the earlier of the twelve (12) month anniversary of the Effective Date or the date on which her employment is terminated in accordance with the terms of CEO Employment Agreement, but that the term of the CEO Employment Agreement may be renewed by written agreement between Dr. Dinu and the Company. Dr. Dinu’s employment is “at will” and terminable by the Company at any time and for any reason or no reason, including as a result of her death or disability, as provided in the CEO Employment Agreement, and with or without “cause”. Dr. Dinu may terminate her employment with the Company at any time and for any reason or no reason, including with or without “good reason”.
The Company will pay Dr. Dinu a base salary at the initial annualized rate of $470,000 per year, subject to standard deductions and withholdings, or such other rate as may be determined from time to time by the Board or the Compensation Committee (hereinafter referred to as the “CEO Base Salary”). Such CEO Base Salary will be paid in accordance with the Company’s standard payroll practice. The CEO Base Salary will be reviewed annually and Dr. Dinu will be eligible to receive a salary increase annually, during the compensation cycle, in an amount to be determined by the Board or the Compensation Committee in its sole and exclusive discretion. Once adjusted, the new salary will become the CEO Base Salary for purposes of the CEO Employment Agreement.
Dr. Dinu will, in accordance with Company policy and the terms of the applicable plan documents, be eligible to participate in benefits under any executive benefit plan or arrangement which may be in effect from time to time and made available to the Company’s executives or key management employees. Dr. Dinu will be eligible to accrue up to sixty days of paid time off per year, in accordance with the Company’s policies as in effect from time to time. The Company will pay or reimburse all reasonable, customary and necessary business expenses, subject to any maximum annual limit or other restrictions as set by the Board or its designated committee.
For each fiscal year of the Company (“FY”) completed during the term of Dr. Dinu’s employment as Chief Executive Officer, Dr. Dinu shall have the opportunity to earn an annual bonus (“CEO Annual Bonus”) under the executive incentive plan then applicable to executives of the Company generally, as in effect from time to time, with the actual amount of each CEO Annual Bonus being determined by the Board or its designated committee based on the achievement of target objectives established by the Board or its designated committee after consultation with Dr. Dinu, and for which the target of the CEO Annual Bonus is an amount equal to 65% of the annual Base Salary during the specific FY. Any CEO Annual Bonus due to Dr. Dinu will be payable not later than two and one-half months following the close of the FY for which the bonus was earned. Except as otherwise provided in the CEO Employment Agreement, Dr. Dinu must be employed on the date annual bonuses are paid under the Company’s executive incentive plan in order to be eligible to earn an CEO Annual Bonus for the preceding FY.
125
Dr. Dinu shall also have the opportunity to earn a bonus (the “Special Achievement Bonus”) upon completion of acquisitions or sales (in each case, whether by merger, asset purchase or stock purchase, or any other method as approved by the Board), or other special activities that generate value to the Company as recognized by the Board or a designated committee of the Board, including, but not limited to, the Compensation Committee, as being eligible for such special achievement bonus. To the extent any of the mentioned above activities are recognized, in good faith, by the Board (or its designated committee) as being eligible for a Special Achievements Bonus, then Dr. Dinu shall have the right to present a proposed bonus structure to the Board, who shall consider the benefit of the activity to the Company’s stockholders, the nature of the activity, the benefits of the activity to the Company’s technology, business development, or cash position and such other factors as the Board and Dr. Dinu agree in good faith are relevant and appropriate. Whether any Special Achievement Bonus is paid and the amount of any such bonus, shall be in the sole discretion of the Board (or its designated committee).
The CEO Employment Agreement provides for the grant of equity awards, in a form to be determined, in the amount of 550,000 shares of common stock to Dr. Dinu, pursuant to and subject to the terms of the Company’s 2024 Equity Incentive Plan (the “2024 Plan”), and subject to approval by the Board and the filing of an effective registration statement on Form S-8 by the Company. Any further equity awards shall be at the discretion of the Board or its designation committee. All of the outstanding and unvested awards held by Dr. Dinu shall vest in the event of a change in control, and if the awards require exercise, be exercisable for the duration of the maximum permitted exercise period as set forth in such grant or grants from the Board, and all of the remaining undelivered shares shall be delivered for such awards that are of stock units immediately prior to and contingent upon the change of control, to the extent delivery will not result in adverse Section 409A tax consequences.
In the event of a change in control, the Company will pay Dr. Dinu the following payments, subject to her continued service through the closing of such change of control transaction and her return of a release of claims: (i) to the extent not already paid, the target amount of the CEO Annual Bonus (the “Target Bonus”) for the entire FY in which the change in control occurs, and (ii) a lump sum equal to (A) two (2) years of (a) the CEO Base Salary in effect and (B) the average of the entire CEO Annual Bonuses and Special Achievement Bonuses paid to Dr. Dinu for the two FYs completed prior to the change of control, as applicable, or, if only one such FY has been completed, then based on the amount of the CEO Annual Bonus and the Special Achievement Bonus for such FY (the “CEO Bonus”) (not including however in calculating the Bonus, any Special Achievement Bonus payable for the change of control transaction shall not be included in determining the entire Annual Bonuses and Special Achievement Bonuses). The occurrence of a change of control will trigger the vesting of all outstanding, unvested awards held by Dr. Dinu and a potential tax equalization payment or gross-up payment which would place Dr. Dinu in the same after-tax position as if any excise tax penalty did not apply with respect to compensation received by Dr. Dinu in connection with such change in control. For the purposes of the CEO Employment Agreement, a “change of control” means the occurrence of one or more of the following: (i) any “Person” or “group,” within the meaning of Section 13(d)(3) or 14(d)(2) of the Exchange Act, other than the Company or any of its affiliates, becomes a beneficial owner, directly or indirectly, in one or a series of transactions, of securities representing fifty percent or more of the total number of votes that may be cast for the election of directors of the Company; (ii) the consummation of a merger or consolidation of the Company with any other person (other than a member of the Company and/or its affiliates), other than a merger or consolidation which would result in the voting securities of the Company outstanding immediately prior thereto continuing to represent (either by remaining outstanding or by being converted into voting securities of the surviving entity) more than fifty percent of the total voting power represented by the voting securities of the Company or such surviving entity outstanding immediately after such merger or consolidation; (iii) within twelve months after a tender offer or exchange offer for voting securities of the Company (other than by the Company) the individuals who were directors of the Company immediately prior thereto shall cease to constitute a majority of the Board; or (iv) there occurs a closing of a sale or other disposition by the Company of all or substantially all of the assets of the Company other than to one or more of the Company’s affiliates.
Dr. Dinu would be entitled to receive certain compensation if her employment with the Company is terminated without “cause” or she resigns for “good reason” (as those terms are defined in the CEO Employment Agreement) in the absence of a change of control transaction, including (i) six months’ of her annual base salary then being paid, payable in equal monthly installments, (ii) a final pro-rated bonus for the period of the year that she has worked prior to termination, (iii) additional compensation equal to eighteen months of Dr. Dinu’s base salary then in effect plus two times the amount of the CEO Bonus (with “CEO Bonus” being defined as the average of the entire CEO Annual Bonuses and Special Achievement Bonuses (each, as defined in the CEO Employment Agreement) paid to Dr. Dinu for the two fiscal years completed prior to her termination), payable in a lump sum subject to certain conditions more fully described in the CEO Employment Agreement.
126
In the event that Dr. Dinu’s employment is terminated as a result of her death, the Company shall pay to her estate within sixty days of the date of termination (the “Date of Termination”) (i) the CEO Base Salary earned but not paid as of the Date of Termination and any un-reimbursed business expenses (together, the “CEO Final Compensation”), (ii) twelve months’ CEO Base Salary in effect as of Date of Termination, (iii) the Target Bonus for the FY in which the Date of Termination occurs, and (iv) the full premium health and dental plan coverage for each of Dr. Dinu’s qualified beneficiaries for the later of the expiration of the term of the CEO Employment Agreement or one year following the Date of Termination, or until COBRA is no longer available to such beneficiaries (the “Beneficiary Benefits”).
If Dr. Dinu’s employment is terminated as a result of a disability (as defined in the CEO Employment Agreement), then, in addition to the Final Compensation, payable as a lump sum as of March 15th of the year following the Date of Termination, the Company will pay Dr. Dinu a pro-rated CEO Annual Bonus for the year during which the Date of Termination takes place, as determined by the Board, and the Beneficiary Benefits.
In the event that Dr. Dinu is terminated with “cause” (as is defined in the CEO Employment Agreement), then the Company shall make no payments to Dr. Dinu other than provision of the CEO Final Compensation, payable no later than ten days after the Date of Termination. Any equity in the Company held by Dr. Dinu in such case shall be governed by the terms of the Company’s equity incentive plans.
If the Company terminates Dr. Dinu or Dr. Dinu terminates her employment for any reason, and a change in control has occurred within twelve months prior to the Date of Termination, then subject to Dr. Dinu’s providing a release of claims and compliance with surviving obligations, including confidentiality, the Company shall provide health and dental plan coverage for Dr. Dinu and her beneficiaries for at most two years.
If Dr. Dinu terminates her employment upon sixty days’ notice, other than for “good reason” and a change in control has not occurred, if the Board so elects, the Company will pay her the CEO Base Salary for the initial sixty days of the notice period in accordance with usual payroll practices.
Concurrent with the CEO Employment Agreement and as a condition thereof, Dr. Dinu entered into a Proprietary Information and Inventions Agreement, which relates to the protection of confidential information of the Company and the ownership by the Company of proprietary information and patents and other intellectual property.
Under the CEO Employment Agreement, the Company has agreed to indemnify Dr. Dinu in accordance with the bylaws and articles of organization of the Company in effect at the time indemnification is applicable, with Dr. Dinu agreeing to provide the Company with prompt notice of any actual or threated claim arising out of her employment. The Company shall also provide Dr. Dinu with the same coverage under any directors and officers liability insurance that the Company elects to maintain as it provides to its other executives, and the same as is provided other former executives, after the termination of her employment.
Employment Agreement with Anastas Budagov
On March 18, 2024, the Board approved an employment letter (the “CFO Employment Agreement”) between Mr. Budagov and the Company, effective as of March 12, 2024, governing the terms of Mr. Budagov’s employment by the Company, which the Company and Mr. Budagov then entered into.
Under the terms of the CFO Employment Agreement, Mr. Budagov will be hired on an “at will” basis and shall serve as the Company’s Chief Financial Officer on a full-time basis, performing his duties and responsibilities remotely.
The Company will pay Mr. Budagov a base salary at the initial annualized rate of $380,000 per year, subject to standard deductions and withholdings, or such other rate as may be determined from time to time by the Board or the Compensation Committee (hereinafter referred to as the “CFO Base Salary”). Such CFO Base Salary will be paid in accordance with the Company’s standard payroll practice. The CFO Base Salary will be reviewed annually and Mr. Budagov will be eligible to receive a salary increase annually, during the compensation cycle, in an amount to be determined by the Board or the Compensation Committee in its sole and exclusive discretion. Once adjusted, the new salary will become the CFO Base Salary for purposes of the CFO Employment Agreement.
Mr. Budagov will, in accordance with Company policy and the terms of the applicable plan documents, be eligible to participate in benefits under any executive benefit plan or arrangement which may be in effect from time to time and made available to the Company’s similarly-situated employees. Further, the CFO Employment Agreement provides that the Company will reimburse in accordance with its standard policies any business expenses incurred, including travel to the Company’s offices.
127
Mr. Budagov shall be eligible to earn an annual performance bonus (the “CFO Annual Bonus”) of up to $63,333 less applicable deductions and withholdings in his initial calendar year of employment, with the target CFO Annual Bonus being 40% of the CFO Base Salary in future years. Eligibility will depend upon applicable performance metrics, established by the Company in its sole discretion, and continuous employment on the date the bonus is paid. The Company shall pay a sign-on bonus to Mr. Budagov in the amount of $63,333.00 less applicable deductions and withholdings.
The CFO Employment Agreement provides for the grant of equity awards, in a form to be determined, in the amount of 325,000 shares of common stock to Mr. Budagov, pursuant to and subject to the terms of the 2024 Plan, and subject to approval by the Board and the filing of an effective registration statement on Form S-8 by the Company. One-third of the shares shall vest on February 15, 2025, and the remaining two-thirds shall vest in eight equal quarterly installments thereafter, subject to continued service with the Company through each vesting date. Any further equity awards shall be at the discretion of the Board or its designation committee.
Pursuant to the termination provisions of the CFO Employment Agreement, Mr. Budagov’s employment will terminate upon his death, and the Company may terminate his employment upon his disability (as defined in the CFO Employment Agreement).
If the Company terminates Mr. Budagov for any reason, then the Company shall pay to Mr. Budagov any CFO Base Salary earned through the Date of Termination, unpaid expense reimbursements, unused, accrued paid time off, and any vested benefits under any employee benefit plan (collectively, the “Accrued Benefit”).
If Mr. Budagov is terminated by the Company without cause, or if he terminates his employment for “good reason” (as defined in the CFO Employment Agreement), then the Company shall pay Mr. Budagov his Accrued Benefit. Further, subject to Mr. Budagov’s providing a release of claims and his compliance with surviving and confidentiality obligations, the Company shall also pay him severance in an amount of six months’ CFO Base Salary, paid in equal monthly installments, and any equity awards that were otherwise eligible to vest solely conditioned on continued service through the next scheduled vesting date for such awards shall vest immediately.
Concurrent with the CFO Employment Agreement and as a condition thereof, Mr. Budagov entered into a Proprietary Information and Inventions Agreement, which relates to the protection of confidential information of the Company and the ownership by the Company of proprietary information and patents and other intellectual property.
Director Compensation
The following table sets forth the compensation earned for services performed for us as a director by each member of our Board as of December 31, 2024, other than any directors who are also our NEOs, during the year ended December 31, 2024.
Name | Fees earned or paid in cash (S) | Option Awards ($) (1) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||
Dr. Avi S. Katz(2) Chairman of the Board | 52,490 | 18,920 | — | 71,410 | ||||||||||||||||||||||
Dr. John C. Klock(3) Director (Class III) | 24,226 | 18,920 | — | 43,146 | ||||||||||||||||||||||
Ross Taylor(4) Director (Class II) | 50,269 | 18,920 | — | 69,189 | ||||||||||||||||||||||
Daniel Dickson(5) Director (Class I) | 34,118 | 18,920 | — | 53,038 | ||||||||||||||||||||||
James Greene(6) Director (Class I) | 48,048 | 18,920 | — | 66,968 | ||||||||||||||||||||||
Professor Zeev Weiner(7) Director (Class II) | 46,232 | 18,920 | — | 65,152 | ||||||||||||||||||||||
_______________
(1)Consists of shares of common stock of the Company issuable upon vesting and exercise of stock options. The amounts in this column represent the aggregate grant date fair value computed in accordance with FASB ASC Topic 718.
128
(2)“Fees earned or paid in cash” consists of $24,226 paid in cash in the 2024 fiscal year for services as a director and $28,264 paid in cash in the 2024 fiscal year as consideration for serving as the Chairman of the Board. “Option Awards” consist of 40,000 stock options granted on July 3, 2024 with one-third vesting on February 15, 2025 and remaining two-thirds vesting quarterly over two years thereafter. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option.
(3)“Fees earned or paid in cash” consists of $24,226 paid in cash in the 2024 fiscal year for services as a director. “Option Awards” consist of 40,000 stock options granted on July 3, 2024 with one-third vesting on February 15, 2025 and remaining two-thirds vesting quarterly over two years thereafter. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option.
(4)“Fees earned or paid in cash” consists of $24,226 paid in cash in the 2024 fiscal year for services as a director and $26,043 paid in cash in the 2024 fiscal year as consideration for services on special board committee. “Option Awards” consist of 40,000 stock options granted on July 3, 2024 with one-third vesting on February 15, 2025 and remaining two-thirds vesting quarterly over two years thereafter. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option.
(5)“Fees earned or paid in cash” consists of $24,226 paid in cash in the 2024 fiscal year for services as a director and $9,892 paid in cash in the 2024 fiscal year as consideration for services on special board committee. “Option Awards” consist of 40,000 stock options granted on July 3, 2024 with one-third vesting on February 15, 2025 and remaining two-thirds vesting quarterly over two years thereafter. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option.
(6)“Fees earned or paid in cash” consists of $24,226 paid in cash in the 2024 fiscal year for services as a director and $23,822 paid in cash in the 2024 fiscal year as consideration for services on special board committee. “Option Awards” consist of 40,000 stock options granted on July 3, 2024 with one-third vesting on February 15, 2025 and remaining two-thirds vesting quarterly over two years thereafter. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option.
(7)“Fees earned or paid in cash” consists of $24,226 paid in cash in the 2024 fiscal year for services as a director and $22,006 paid in cash in the 2024 fiscal year as consideration for services on special board committee. “Option Awards” consist of 40,000 stock options granted on July 3, 2024 with one-third vesting on February 15, 2025 and remaining two-thirds vesting quarterly over two years thereafter. The stock options have an exercise price of $0.748 per option and a grant date fair value of $0.47 per option.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of our common stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or executive officer when entering into the plan, without further direction from them. The director or executive officer may amend a Rule 10b5-1 plan in some circumstances and may terminate a plan at any time. Our directors and executive officers also may buy or sell additional shares outside of a Rule 10b5-1 plan when they are not in possession of material nonpublic information, subject to compliance with the terms of our insider trading policy.
Emerging Growth Company Status
We are an “emerging growth company,” as defined in the JOBS Act. As an emerging growth company, we are exempt from certain requirements related to executive compensation, including the requirements to hold a nonbinding advisory vote on executive compensation and to provide information relating to the ratio of total compensation of its chief executive officer to the median of the annual total compensation of all of its employees, each as required by the Investor Protection and Securities Reform Act of 2010, which is part of the Dodd-Frank Wall Street Reform and Consumer Protection Act.
Item 12: Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth information as of March 28, 2025 regarding the beneficial ownership of shares of common stock of the Company by:
129
•each person, including any “group” as defined in Section 13(d)(3) of the Exchange Act, known to be the beneficial owner of more than 5% of the common stock of the Company;
•each of the Company’s NEOs as of December 31, 2024;
•each of the Company’s directors as of March 28, 2025; and
•all of the Company’s NEOs as of December 31, 2024 and directors as of March 25, 2024, as a group.
Beneficial ownership is determined according to the rules of the SEC, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days. As of March 25, 2024, there were 27,653,210 shares of our common stock outstanding.
Unless otherwise indicated, the Company believes that all persons named in the table have sole voting and investment power with respect to all shares of common stock of the Company beneficially owned by them.
| Name and Address of Beneficial Owner† | Number of Shares of Common Stock Beneficially Owned | Percentage of Outstanding Common Stock % | ||||||||||||
Directors and Named Executive Officers: | ||||||||||||||
Dr. Avi S. Katz (2)(3)(4)(5) | 1,360,335 | 4.8 | % | |||||||||||
Dr. John Klock (1)(4)(6) | 2,894,473 | 10.5 | % | |||||||||||
Dr. Raluca Dinu (1)(3)(7) | 1,518,561 | 5.3 | % | |||||||||||
Ross Taylor (1)(3)(4) | 355,797 | 1.3 | % | |||||||||||
Professor Zeev Weiner (1)(3)(4) | 184,565 | * | ||||||||||||
Daniel Dickson (1)(3)(4) | 184,565 | * | ||||||||||||
James Greene (1)(3)(4)(8) | 1,123,497 | 4.0 | % | |||||||||||
Anastas Budagov (1)(9) | 108,333 | * | ||||||||||||
All Directors and Executive Officers of the Company as a Group (8 Individuals) | 7,730,126 | 25.8 | % | |||||||||||
Five Percent or Greater Holders: | ||||||||||||||
John C. Klock, Jr. and Cynthia L. Klock Trust Dated 7/27/07(1)(4)(6) | 2,894,473 | 10.5 | % | |||||||||||
Lynrock Lake Master Fund LP(10) | 2,666,245 | 9.6 | % | |||||||||||
Emil D. Kakkis and Jenny Soriano(11) | 1,400,300 | 5.1 | % | |||||||||||
__________________
*Less than 1%.
(1)Unless otherwise indicated, the business address of each of the individuals is 3 Hamilton Landing, Suite 160, Novato, CA 94949.
(2)The business address for this person is 1731 Embarcadero Road, Suite 200, Palo Alto, California 94303.
(3)Includes shares of common stock underlying warrants that are exercisable within 60 days.
(4)Includes 13,333 shares of common stock underlying stock options that are exercisable within 60 days.
(5)Includes warrants to purchase 571,431 shares of common stock that are exercisable within 60 days.
(6)Shares held by John C. Klock, Jr. and Cynthia L. Klock Trust Dated 7/27/07.
(7)Includes 546,325 shares of common stock underlying warrants and 196,666 shares of common stock underlying stock options that are exercisable within 60 days.
(8)Includes 200,000 shares of common stock and 428,082 shares of common stock underlying warrants held by Sky D Ventures, LLC that are exercisable within 60 days. The securities held by Sky D Ventures, LLC are beneficially owned by James Greene, a member of our Board. Mr. Greene is also the Managing Member of Sky D Ventures, LLC, who has sole voting and dispositive power over the securities held by Sky D Ventures, LLC.
(9)Consists of 108,333 shares of common stock underlying stock options that are exercisable within 60 days.
(10) Shares held by Lynrock Lake Master Fund LP. Lynrock Lake LP is the investment manager of Lynrock Lake Master Fund LP, and pursuant to an investment management agreement, Lynrock Lake LP has been delegated full voting and investment power over the shares held by Lynrock Lake Master Fund LP. Cynthia Paul, the Chief Investment Officer of Lynrock Lake LP and Sole Member of Lynrock Lake Partners LLC, the general partner of Lynrock Lake LP, may be deemed to exercise voting and investment power over the shares held by Lynrock Lake Master Fund LP. Pursuant to the terms of the Warrant Agreement that governs certain of the warrants for the purchase of shares of common stock held by Lynrock Lake Master Fund LP, Lynrock Lake Master Fund LP has elected to not have the right to exercise such warrant, to the extent that after giving effect to such exercise, it would beneficially own in excess of 4.9% of the shares of common stock issued and outstanding immediately after giving effect to such exercise. Pursuant to the terms of the other Warrant held by Lynrock Lake Master Fund LP, Lynrock Lake Master Fund LP has elected to not have the right to exercise such warrant, to the extent that after giving effect to such
130
exercise, it would beneficially own in excess of 4.99% of the shares of common stock issued and outstanding immediately after giving effect to such exercise. The address of the foregoing entities is c/o Lynrock Lake LP, 2 International Drive, Suite 130, Rye Brook, New York 10573.
(11)The business address for this group is 60 Leveroni Court, Novato, California 94949.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table presents information as of December 31, 2024 regarding equity compensation plans under which the Company’s equity securities are authorized for issuance:
Plan Category | Number of Securities to be Issued upon Exercise of Outstanding Options | Weighted-Average Exercise Price of Outstanding Options | Number of Securities Remaining Available for Future Issuance under Equity Compensation Plans | |||||||||||||||||
Equity compensation plans approved by security holders | 2,219,000 | $ | 0.72 | 139,093 | ||||||||||||||||
Equity compensation plans not approved by security holders | — | — | — | |||||||||||||||||
Totals | 2,219,000 | 139,093 | ||||||||||||||||||
Item 13: Certain Relationships and Related Transactions, and Director Independence
GigCapital5 Related Agreements
GigCapital5 Registration Rights Agreement
In connection with the Business Combination, at the closing, the Company, GigAcquisitions5 and certain securityholders of GigCapital5 and QT Imaging entered into the GigCapital5 Registration Rights Agreement. In addition, pursuant to the terms of the GigCapital5 Registration Rights Agreement and subject to certain requirements and customary conditions, such securityholders may demand at any time or from time to time, that the Company file a registration statement on Form S-3 (or any similar short-form registration which may be available) to register the resale of the registrable securities of the Company held by such securityholders. The GigCapital5 Registration Rights Agreements provides these securityholders (and their permitted transferees) with the right to require the Company, at the Company’s expense, to register shares of common stock that they hold on customary terms, including customary demand and piggyback registration rights. The GigCapital5 Registration Rights Agreement also provides that the Company pay certain expenses of the electing holders relating to such registrations and indemnify them against certain liabilities that may arise under the Securities Act.
Under the GigCapital5 Registration Rights Agreement, the Company will indemnify such securityholders and certain persons or entities related to such securityholders such as their officers, employees, directors, and agents against any losses or damages resulting from any untrue or alleged untrue statement, or omission or alleged omission, of a material fact in any registration statement or prospectus pursuant to which the securityholders sell their registrable securities, unless such liability arose from such securityholder’s misstatement or alleged misstatement, or omission or alleged omission, and the securityholders including registrable securities in any registration statement or prospectus will indemnify the Company and certain persons or entities related to the Company such as its officers and directors and underwriters against all losses caused by their misstatements or omissions (or alleged misstatements or omissions) in those documents.
The Company registered securities for resale in accordance with the terms of the GigCapital5 Registration Rights Agreement in a registration statement on Form S-1 that was declared effective by the SEC on May 22, 2024.
Lock-up Agreement
GigCapital5 and certain stockholders of the Company entered into a lock-up agreement (the “Lock-Up Agreement”) at the closing of the Business Combination. The Lock-Up Agreement provided that, subject to certain exceptions, each of
131
such stockholders will not transfer any shares of the common stock beneficially owned or owned of record by such of the stockholders until the earlier of (a) six months following the closing Date; (b) subsequent to the closing, the date on which the reported closing price of one share of the common stock quoted on the Nasdaq equals or exceeds $11.50 per share (as adjusted for stock splits, stock dividends, reorganizations, recapitalizations and the like occurring after the closing) for any twenty trading days within any thirty consecutive trading day period commencing at least ninety days after the closing Date; and (c) subsequent to the closing, the date on which the Company completes a liquidation, merger, stock exchange or other similar transaction that results in all of the Company’s stockholders having the right to exchange their Company securities for cash, securities or other property. The restrictions on transfer under the Lock-Up Agreement expired on September 4, 2024.
Working Capital Notes
On December 13, 2023, GigCapital5 issued that certain Eleventh Amended and Restated Working Capital Note (the “Working Capital Note”) to GigAcquisitions5 for an aggregate principal amount of $1,500,000, the terms of which provide that GigAcquisitions5 may elect to convert the Working Capital Note, at a price of $10.00 per unit, into units identical to the private placement units issued in connection with GigCapital5’s initial public offering. In connection with the closing, (i) GigAcquisitions5 elected to partially convert (the “Conversion”) $943,640 in principal balance outstanding under the Working Capital Note into 94,364 shares of common stock and 94,364 GigAcquisitions5’s private warrants of the Company, and (ii) the Company repaid the remaining principal balance of $556,360 to GigAcquisitions5 concurrently with the Conversion, such that the Company’s obligations under the Working Capital Note have been satisfied in full. In addition, on December 13, 2023, GigAcquisitions5 made an additional, unsecured, loan in the principal amount of $66,360 to GigAcquisitions5 (the “Non-Convertible Working Capital Note”). The Non-Convertible Working Capital Note was issued to provide the Company with additional working capital and was not deposited into the Trust Account. On February 7, 2024, the Company amended and restated the Non-Convertible Working Capital Note (the “Second Non-Convertible Working Capital Note”) to reflect an additional principal amount of $195,887 extended by GigAcquisitions5 to the Company for a collective principal amount under the Second Non-Convertible Working Capital Note of $262,247. The Second Non-Convertible Working Capital Note was issued to provide the Company with additional working capital and was not deposited into the Trust Account. The Company issued the Second Non-Convertible Working Capital Note in consideration for an additional loan from GigAcquisitions5 to fund the Company’s working capital requirements.
Extension Note
On August 28, 2023, GigCapital5 issued that certain non-convertible Eleventh Amended and Restated Promissory Note (as amended, the “Extension Note”) to GigAcquisitions5 for an aggregate principal amount of $1,560,000. On March 4, 2024, the Company and GigAcquisitions5 agreed to amend and restate the Extension Note to extend the date of maturity until March 4, 2025. On November 22, 2024, GigAcquisitions5 exchanged the Extension Note for the purchase of PIPE Shares and PIPE Warrants in the Private Placement.
QT Imaging Related Agreements
QTI Working Capital Note
In July 2020, QT Imaging, Inc. issued a convertible promissory note to Dr. John Klock that was amended on September 1, 2022 and November 14, 2022 (the "2022 Klock Note"), for a principal amount of $2,643,725. In addition, on May 3, 2023, QT Imaging, Inc. issued a promissory note (the “QTI Working Capital Note”) to Dr. Klock for a principal amount of $250,000. The QTI Working Capital Note was subsequently amended and restated five times on June 12, 2023 to add an additional principal amount of $100,000, August 15, 2023 to add an additional principal amount of $75,000, August 29, 2023 to add an additional principal amount of $100,000, September 12, 2023 to add an additional principal amount of $75,000, and September 15, 2023 to add an additional principal amount of $50,000, for an aggregate principal amount outstanding as of September 30, 2023 under the QTI Working Capital Note of $650,000. The QTI Working Capital Note was issued to provide the Company with additional working capital during the period prior to consummation of the business combination agreement with GigCapital5, Inc. The QTI Working Capital Note is interest-free and matures on the earlier of (i) the date on which the Company consummates the business combination with GigCapital5, Inc.; (ii) the date the Company winds up; or (iii) December 1, 2023. The QTI Working Capital Note may be prepaid without penalty. On October 26, 2023, the QTI Working Capital Note was amended to increase the outstanding principal amount to $705,000 and extend the potential maturity date from December 1, 2023 to December 31, 2023. In connection with the closing of the Business Combination, the 2022 Klock Note and the QTI Working Capital Note maturity date was extended to July 1, 2025, the Company assumed the obligations under both of these notes, and all security interests and liens in favor of Dr. Klock were removed. In connection with the issuance of the note to Lynrock Lake, on February 26, 2025, the maturity dates on both of these notes held by Dr. Klock were extended to October 21, 2027.
132
QT Imaging Holdings Agreements
Dr. Klock has previously operated a medical practice as a sole proprietorship under the name QT Imaging Center (the “Practice”), where, among other things, patients can receive scans using a QT Breast Scanner. Dr. Klock retired effective December 31, 2024, and notified the Company that he is no longer operating the Practice.
Data Use and License Agreement
On April 3, 2024, the Company entered into a Data Use and License Agreement with the Practice, that conducts a medical practice and provides medical services, pursuant to which the Company was granted a license to use and disclose certain de-identified health information, as has been de-identified by the Practice in accordance with applicable law, for use in research and analytical processes in connection with the Company’s development and commercialization of the QT Ultrasound Breast Scanner-1 and other technologies.
Services Agreement
On April 5, 2024, the Company entered into a services agreement (the “Services Agreement”) with the Practice dated as of April 1, 2024 pursuant to which the Practice agreed to provide its services to the Company, including but not limited to providing healthcare services to patients, assisting with clinical trials and studies and assisting with drafting of institutional review board approved clinical protocols, assisting with the performance of research and development activities on behalf of the Company, providing comprehensive multi-day training on the operation of breast imaging technology for radiologist customers and other customer staff such as technicians, performing clinical validation of imaging software changes which may include recruiting patients, training of NXC personnel or Canon or its affiliates personnel on the operation of the Company’s imaging technology, as well as other services as specified in the Services Agreement. The Practice will receive $450 per hour for these services to be performed by Dr. Klock for a minimum of 15 hours a week as needed by the Company and its business and technical partners and not to exceed 60 hours per month (unless requested by the Company and agreed to by Dr. Klock). The parties have agreed that this compensation is the fair market value for the professional time of Dr. Klock, without taking into consideration the volume of value of any referrals of business between the parties. The QT Imaging Center will submit to the Company a written report listing the deliverables and the work hours (in increments of one quarter hour) rendered by the Practice during the previous three calendar months (the “Quarterly Report”) no later than five business days following the end of the last calendar month included in the Quarterly Report. The Company shall pay the compensation for the services to the Practice on a quarterly basis no later than fifteen business days after the month of the Company’s receipt of the Quarterly Report, unless there is a dispute concerning the Quarterly Report, in which case the Company shall timely communicate such dispute to the Practice. The term of the Services Agreement is one year unless earlier terminated and shall auto-renew for successive one-year periods, unless otherwise terminated. As a result of Dr. Klock's retirement on December 31, 2024, the Services Agreement has terminated.
Space and Equipment Sublease
On April 17, 2024, the Company, entered into a space and equipment sublease agreement (the “Space and Equipment Sublease”) with the Practice, pursuant to which the Practice will sublease certain medical equipment and space, currently leased from Hamilton Landing Novato LLC by the Company, to the Practice for use in its operations, on a full-time and exclusive basis. The Practice shall pay to the Company the rent for the Subleased Space (as defined in the Space and Equipment Sublease) on a monthly basis, payable on the first day of each month and no later than ten days thereafter, with the rent to be pro-rated for any partial month. The parties have determined that the rent equals the fair market value of the Subleased Space and subleased equipment (as defined in the Space and Equipment Sublease), without taking into account the proximity of the parties or the space to any source, volume or value of referrals between the parties or any patient thereof. Further, the Practice shall pay when due all sales, use, personal property, leasing, excise or other fees, taxes, charges or withholdings of any kind imposed against the Company, the Practice or the subleased equipment with respect to the Space and Equipment Sublease, the subleased equipment, or any related fees, receipts or earnings, including local taxes and personal property taxes. As a result of Dr. Klock's retirement on December 31, 2024, the Space and Equipment Sublease has terminated.
Sublease Agreement
Subsequent to his December 31, 2024 retirement, Dr. Klock informed the Company that he would continue the Practice and that he would like to enter into a sublease for the Subleased Space. On January 23, 2025, the Company, entered into a Sublease Agreement (the “Sublease”) with the Practice for a sublease of the Subleased Space. The Practice shall pay to the Company a rental fee (the “Rent”) for the Subleased Space (as defined in the Sublease) in an amount equal
133
to $5,666.00 until May 31, 2025, with such amount increasing to $5,836.24 during the period from June 1, 2025 until May 31, 2026, and subsequently $6,011.33 during the period from June 1, 2026 until May 31, 2027. The Rent shall be payable on a monthly basis, payable on the first day of each month and no later than five days thereafter, with the Rent to be pro-rated for any partial month. The parties have determined that the Rent equals the fair market value of the Subleased Space (as defined in the Sublease), without taking into account the proximity of the parties or the Subleased Space to any source, volume or value of referrals between the parties or any patient thereof. Further, the Practice shall pay when due all sales, use, personal property, leasing, excise or other fees, taxes, charges or withholdings of any kind imposed against the Company, the Practice or the Subleased Space with respect to the Subleased Space, or any related fees, receipts or earnings, including local taxes and personal property taxes. The term of the Sublease is one year unless terminated and shall auto-renew on a month-to-month basis thereafter, unless otherwise terminated. The Sublease shall expire automatically upon the termination of the Prime Lease, which is set to terminate in April 2027.
Related Person Transactions Policy
The Board has adopted a related person transaction policy that sets forth the Company’s procedures for the identification, review, consideration and approval or ratification of related person transactions. The policy became effective upon approval by the Board following the consummation of the Business Combination. The Company’s Audit Committee has the primary responsibility for reviewing and approving or disapproving “related party transactions.” The charter of the Company’s Audit Committee provides that the Audit Committee will review and approve in advance any related party transaction.
A related person transaction is a transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships, between the Company and related persons in which the aggregate amount involved exceeds or may be expected to exceed $120,000 and in which a related person has or will have a direct or indirect material interest. Transactions involving compensation for services provided to the Company as an employee or director are not expected to be covered by this policy. A related person is any executive officer, director or beneficial owner of more than 5% of any class of the Company’s voting securities and any of their respective immediate family members and any entity owned or controlled by such persons.
It is expected that under the policy, if a transaction has been identified as a related person transaction, including any transaction that was not a related person transaction when originally consummated or any transaction that was not initially identified as a related person transaction prior to consummation, the Company’s management must present information regarding the related person transaction to the Company’s Audit Committee, or, if Audit Committee approval would be inappropriate, to another independent committee of the Board, for review, consideration and approval or ratification. The presentation must include a description of, among other things, the material facts, the interests (direct and indirect) of the related persons, the benefits to the Company of the transaction and whether the transaction is on terms that are comparable to the terms available to or from (as the case may be) an unrelated third party or to or from employees generally. Under the policy, the Company will collect information that it deems reasonably necessary from each director, executive officer and, to the extent feasible, significant stockholder to enable the Company to identify any existing or potential related person transactions and to effectuate the terms of the policy. In addition, under the Company’s Code of Business Conduct and Ethics, the Company’s employees and directors have an affirmative responsibility to disclose any transaction or relationship that reasonably could be expected to give rise to a conflict of interest. In considering related person transactions, it is expected that the Company’s Audit Committee, or other independent committee of the Board, will take into account the relevant available facts and circumstances including, but not limited to:
•the risks, costs and benefits to the Company;
•the impact on a director’s independence in the event that the related person is a director, immediate family member of a director or an entity with which a director is affiliated;
•the availability of other sources for comparable services or products; and
•the terms available to or from, as the case may be, unrelated third parties or to or from employees generally.
The policy also requires that, in determining whether to approve, ratify or reject a related person transaction, the Company’s Audit Committee, or other independent committee of the Board, will consider, in light of known circumstances, whether or not the transaction is consistent with the Company’s best interests and those of the Company’s stockholders, as the Company’s Audit Committee, or other independent committee of the Board, determines in the good faith exercise of its discretion.
Director Independence
134
The Board has undertaken a review of the independence of each director. Based upon information requested from and provided by each director concerning their background, employment, and affiliations, including family relationships, the following members of the Board do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and the Board has determined that each of these directors is “independent” as that term is defined under Nasdaq rules: Ross Taylor, Daniel Dickson, James Greene and Professor Zeev Weiner.
In making these determinations, the Board has considered the current and prior relationships that each non-employee director has with the Company and all other facts and circumstances that the Board deems relevant in determining their independence, including the beneficial ownership of the Company’s capital stock by each non-employee director, and the transactions involving them described in the section titled “Certain Relationships and Related Transactions, and Director Independence.” Other than that Drs. Katz and Dinu are married to each other, there are no family relationships among any of the directors or executive officers of the Company.
Item 14: Principal Accountant Fees and Services
The following is a summary of fees billed by BPM LLP (“BPM”) for the 2023 and 2024 fiscal years:
Fiscal Year 2023 | Fiscal Year 2024 | ||||||||||||||||
Audit Fees (1) | $ | 337,666 | $ | 406,470 | |||||||||||||
Audit-Related Fees (2) | — | — | |||||||||||||||
Tax Fees (3) | — | — | |||||||||||||||
All Other Fees (4) | — | — | |||||||||||||||
Total | $ | 337,666 | $ | 406,470 | |||||||||||||
(1) Audit fees consist of fees billed and to be billed for professional services rendered for the audit of our year-end financial statements, reviews of our condensed financial statements and services that are normally provided by our independent registered public accounting firm in connection with statutory and regulatory filings.
(2) Audit-related fees consist of fees billed for assurance and related services that are reasonably related to performance of the audit or review of our year-end financial statements and are not reported under “Audit Fees.” These services include attest services that are not required by statute or regulation and consultation concerning financial accounting and reporting standards, including permitted due diligence services related to a potential business combination.
(3) Tax fees consist of fees billed for professional services relating to tax compliance, tax planning and tax advice.
(4) All other fees consist of fees billed for all other services.
Policy on Board Pre-Approval of Audit and Permissible Non-Audit Services of the Independent Auditors
The Audit Committee is responsible for appointing, setting compensation and overseeing the work of the independent registered public accounting firm. In recognition of this responsibility, the Audit Committee shall review and, in its sole discretion, pre-approve all audit and permitted non-audit services to be provided by the independent registered public accounting firm as provided under the Audit Committee charter.
The Audit Committee has determined that all services performed by BPM are compatible with maintaining the independence of BPM. The Audit Committee’s policy is to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm. These services may include audit services, audit-related services, tax services and other services. The Audit Committee has delegated to the Chairman of the Audit Committee the authority to approve permitted services provided that the Chairman reports any decisions to the Audit Committee at its next scheduled meeting. The independent registered public accounting firm and management are required to periodically report to the Audit Committee regarding the extent of services provided by the independent registered public accounting firm in accordance with this pre-approval process.
135
Part IV
Item 15. Exhibits and Financial Statement Schedules.
(a)Exhibits.
| Exhibit No. | Description | |||||||
2.1†* | ||||||||
2.2* | ||||||||
2.3†* | ||||||||
2.4* | ||||||||
2.5* | ||||||||
2.6* | ||||||||
3.1* | ||||||||
3.2* | ||||||||
4.1* | ||||||||
4.2* | ||||||||
4.3* | ||||||||
| 10.1* | ||||||||
| 10.2* | ||||||||
| 10.3* | ||||||||
| 10.4#* | ||||||||
| 10.5* | ||||||||
136
| 10.6†* | ||||||||
| 10.7* | ||||||||
| 10.8#* | ||||||||
| 10.9* | ||||||||
| 10.10* | ||||||||
| 10.11* | ||||||||
| 10.12* | ||||||||
| 10.13* | ||||||||
| 10.14* | ||||||||
| 10.15* | ||||||||
| 10.16* | ||||||||
| 10.17* | ||||||||
| 10.18* | ||||||||
| 10.19* | ||||||||
| 10.20†* | ||||||||
10.21#* | ||||||||
10.22#* | ||||||||
10.23#* | ||||||||
137
| 10.24* | ||||||||
| 10.25* | ||||||||
| 10.26* | ||||||||
| 10.27* | ||||||||
| 10.28†* | ||||||||
| 10.29* | ||||||||
| 10.30†* | ||||||||
| 10.31#* | ||||||||
| 10.32* | ||||||||
| 10.33* | ||||||||
| 10.34* | ||||||||
| 10.35* | ||||||||
| 10.36* | ||||||||
| 10.37* | ||||||||
| 10.38* | ||||||||
| 10.39* | ||||||||
138
| 10.40* | ||||||||
10.41* | ||||||||
10.42* | ||||||||
10.43* | ||||||||
10.44* | ||||||||
10.45* | ||||||||
| 10.46†** | ||||||||
| 10.47†** | ||||||||
| 14* | ||||||||
19.1** | ||||||||
21.1* | ||||||||
23.1** | ||||||||
24** | Power of Attorney (included on signature page to initial filing of this Annual Report). | |||||||
31.1** | ||||||||
31.2** | ||||||||
32.1*** | ||||||||
32.2*** | ||||||||
| 97.1* | ||||||||
| 101.INS** | Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document. | |||||||
| 101.CAL** | Inline XBRL Taxonomy Extension Calculation Linkbase Document. | |||||||
| 101.SCH** | Inline XBRL Taxonomy Extension Schema Document. | |||||||
| 101.DEF** | Inline XBRL Taxonomy Extension Definition Linkbase Document. | |||||||
| 101.LAB** | Inline XBRL Taxonomy Extension Labels Linkbase Document. | |||||||
| 101.PRE** | Inline XBRL Taxonomy Extension Presentation Linkbase Document. | |||||||
| 104** | Cover Page Interactive Data File (as formatted as Inline XBRL and contained in Exhibit 101). | |||||||
___________________
*Previously filed and incorporated herein by reference.
**Filed herewith.
*** Furnished herewith.
139
†Certain portions of this exhibit (indicated by “[***]”) have been omitted pursuant to Item 601(b)(10)(iv) of Regulation S-K because it is not material and is the type of information that the Registrant treats as private or confidential. The Registrant agrees to furnish supplementally a copy of such schedules, or any section thereof, to the SEC upon request.
#Indicate management contract or compensatory plan or arrangement.
Item 16: Form 10-K Summary
None.
140
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of Novato, State of California on March 31, 2025.
| QT IMAGING HOLDINGS, INC. | |||||
| /s/ Dr. Raluca Dinu | |||||
| Name: Dr. Raluca Dinu | |||||
| Title: Chief Executive Officer | |||||
141
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each of the undersigned constitutes and appoints Dr. Raluca Dinu, acting alone, his or her true and lawful attorney-in-fact and agents, with full power of substitution and resubstitution, for such person and in his or her name, place and stead, in any and all capacities, to sign this Registration Statement on Form S-1 (including all pre-effective and post-effective amendments and registration statements filed under the Securities Act of 1933), and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming that such attorney-in-fact and agent, or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Position | Date | ||||||||||||
/s/ Dr. Raluca Dinu | Chief Executive Officer and Director (Principal Executive Officer) | March 31, 2025 | ||||||||||||
Dr. Raluca Dinu | ||||||||||||||
/s/ Anastas Budagov | Chief Financial Officer (Principal Financial and Accounting Officer) | March 31, 2025 | ||||||||||||
Anastas Budagov | ||||||||||||||
/s/ Dr. John Klock | Director | March 31, 2025 | ||||||||||||
| Dr. John Klock | ||||||||||||||
/s/ Dr. Avi Katz | Chairman of the Board of Directors | March 31, 2025 | ||||||||||||
Dr. Avi Katz | ||||||||||||||
/s/ Ross Taylor | Director | March 31, 2025 | ||||||||||||
Ross Taylor | ||||||||||||||
/s/ Daniel Dickson | Director | March 31, 2025 | ||||||||||||
| Daniel Dickson | ||||||||||||||
/s/ James Greene | Director | March 31, 2025 | ||||||||||||
| James Greene | ||||||||||||||
/s/ Prof. Zeev Weiner | Director | March 31, 2025 | ||||||||||||
| Prof. Zeev Weiner | ||||||||||||||
142