
Quantitative Transmission Imaging Breast Acoustic CT Scanner INVESTOR PRESENTATION May 2025

2 Disclaimer ABOUT THIS PRESENTATION This investor presentation (this “Presentation”) is provided for informational purposes only. The information contained herein does not purport to be all-inclusive and neither QT Imaging Holdings, Inc. (the “Company”, “QT Imaging Holdings”, “QTI”), nor its respective directors, officers, employees, agents, advisors or affiliates, including QT Imaging, Inc. (“QT Imaging”), makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this Presentation, which has not been verified and is subject to change at any time. Viewers of this Presentation should each make their own evaluation of QT Imaging Holdings and of the relevance and accuracy of the information and should make such other investigations as they deem necessary. To the fullest extent permitted by law, no responsibility or liability whatsoever is accepted by QT Imaging Holdings, or its directors, officers, employees, agents, advisors or affiliates for any loss howsoever arising, directly or indirectly, from any use of this Presentation or such information or opinions contained herein or otherwise arising in connection herewith. This Presentation does not constitute (i) a solicitation of a proxy, consent or authorization with respect to any securities or (ii) an offer to sell, a solicitation of an offer to buy, or a recommendation to purchase any security of QT Imaging Holdings, or any of its affiliates, nor shall there be any sale, issuance or transfer of securities in any jurisdiction where, or to any person to whom, such offer, solicitation or sale would be unlawful. You should not construe the contents of this Presentation as legal, tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein, and, by accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision. On June 6, 2017, the U.S. Food and Drug Administration ("FDA") in response to QT Imaging’s Section 510(k) Summary of Safety and Effectiveness premarket notification under the Food, Drug and Cosmetic Act, determined that the QT Breast Scanner is substantially equivalent to the predicate device. Our use of the words “safe”, “safety”, “effectiveness”, and “efficacy” in relation to the QT Breast Scanner in this Presentation and all other QT Imaging related documents is limited to the context of the Section 510(K) Summary of Safety and Effectiveness that was reviewed and responded to by the FDA. TRADEMARKS AND INTELLECTUAL PROPERTY All trademarks, service marks, and trade names of QT Imaging Holdings or its affiliates used herein are trademarks, service marks, or registered trade names of QT Imaging Holdings or its affiliates, as noted herein. Any other product, company names, or logos mentioned herein are the trademarks and/or intellectual property of their respective owners, and their use is not intended to, and does not imply, a relationship with QT Imaging Holdings or its affiliates, or an endorsement or sponsorship by or of QT Imaging Holdings or its affiliates. Solely for convenience, the trademarks, service marks and trade names referred to in this presentation may appear without the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that QT Imaging Holdings or its affiliates will not assert, to the fullest extent under applicable law, their rights or the right of the applicable licensor to these trademarks, service marks and trade names. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

3 Disclaimer FORWARD LOOKING STATEMENTS Certain statements included in this Presentation that are not historical facts are forward-looking statements for purposes of the safe harbor provisions under the United States Private Securities Litigation Reform Act of 1995. Forward-looking statements generally are accompanied by words such as “has the potential to”, “believe”, “may”, “will”, “estimate”, “continue”, “anticipate”, “intend”, “expect”, “should”, “would”, “plan”, “predict”, “potential”, “seem”, “seek”, “future”, “outlook”, and similar expressions that indicate or predict future events or trends that are not statements of historical matters. These forward looking statements include, but are not limited to, the potential impact on existing medical technology, the company’s technology, products, business prospects, revenue, client adoptions, commercialization, projections of market opportunity and statements regarding estimates and forecasts of other financial and performance metrics. These statements are based on various assumptions, whether or not identified in this Presentation, and on the current expectations of QT Imaging Holdings’ management and are not predictions of actual performance. These forward-looking statements are provided for illustrative purposes only and are not circumstances intended to serve as, and must not be relied on by any investor as, a guarantee, an assurance, a prediction or a definitive statement of fact or probability. In addition, statements regarding the Company’s products, technology, and market opportunity reflect the beliefs and opinions of QT Imaging Holdings’ management on the relevant subject as of this Presentation. Actual events and circumstances are difficult or impossible to predict and will differ from assumptions. Many actual events and circumstances are beyond the control of QT Imaging Holdings. These forward-looking statements are subject to a number of risks and uncertainties, including changes in domestic and foreign business, market, financial, political and legal conditions; risks related to the rollout of QT Imaging Holdings’ business and the timing of expected business milestones; the demand for QT Imaging Holdings’ products and services; the ability of QT Imaging Holdings to increase sales of its output products in accordance with its plans; issues that could arise with respect to the manufacture of QT scanners by CMSC; the desire of customers and service recipients to continue engaging QT Imaging Holdings; the effects of competition on QT Imaging Holdings’ future business, changes in the Company’s strategy, future operations, financial positions, and product development timeline. If any of these risks materialize or our assumptions prove incorrect, actual results could differ materially from the results implied by these forward-looking statements. There may be additional risks that QT Imaging Holdings presently does not know or believes is immaterial that could also cause actual results to differ from those contained in the forward-looking statements. In addition, forward-looking statements reflect QT Imaging Holdings’ expectations, plans or forecasts of future events and views as of the date of this Presentation. QT Imaging Holdings anticipates that subsequent events and developments will cause its assessments to change. However, while QT Imaging Holdings may elect to update these forward-looking statements at some point in the future, its specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing QT Imaging Holdings’ assessments as of any date subsequent to the date of this Presentation. Accordingly, undue reliance should not be placed upon the forward-looking statements. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

4 Disclaimer NON-GAAP FINANCIAL MEASURES This presentation includes references to EBITDA and Adjusted EBITDA, financial measures that have not been prepared in accordance with generally accepted accounting principles in the United States (“GAAP”). EBITDA is defined as loss before interest expense, income tax expense, depreciation and amortization. Adjusted EBITDA is defined as EBITDA further adjusted for equity-based compensation, net change in fair value of the derivative, earnout and warrant liabilities, and transaction expenses. Similar excluded expenses may be incurred in future periods when calculating these measures. QT Imaging believes these non-GAAP measures of financial results provide useful information to management and investors regarding certain financial and business trends relating to the Company’s financial condition and results of operations. QT Imaging believes that the use of these non-GAAP financial measures provides an additional tool for investors to use in evaluating projected operating results and trends and in comparing QT Imaging’s financial measures with other similar companies, many of which present similar non-GAAP financial measures to investors. Investors should not rely on any single financial measure to evaluate QT Imaging’s anticipated business. Certain of the financial metrics in this presentation can be found in QT Imaging’s Form 8-K filed with the U.S. Securities and Exchange Commission (the “SEC”) on May 13, 2025, and the reconciliation of EBITDA and Adjusted EBITDA can be found on pages 65 and 66 of this presentation. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

5 QT Imaging Holdings (QTI) Has the Potential to Transform Medical Imaging • QTI is a medical device company with imaging technology that has the potential to transform the industry • QTI Scanner is the only 3D imaging device to receive FDA clearance for use as a transmission and reflection ultrasonic imaging system of a patient’s breast • QTI’s patent-protected technology provides a high resolution, relatively low-cost, comprehensive, no radiation, no discomfort medical imaging solution • QTI’s technology yields improved diagnostic performance compared to traditional mammogram and has similar imaging quality compared to MRI but is a lower cost and more accessible solution. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

6 Our Mission • Create disruptive innovation using technology (software, machine learning, and smart physics) to improve medical imaging and thus, healthcare quality and access • Continue to build upon our FDA clearances to offer QTI as a breast screening imaging modality • Expand the market opportunities beyond hospitals, imaging centers and health centers by supporting additional direct to consumer (DTC) and direct to provider (DTP) approaches • Introduce the first comprehensive body-safe imaging technology, enabling for the first-time well-person body imaging health screening NIH has awarded QT Imaging about $18M for new women’s imaging solution Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

7 Our Management Team Stas Budagov CHIEF FINANCIAL OFFICER Nasser C. Pirshafiey, MBA CHIEF PRODUCT OFFICER Dr. Raluca Dinu CHIEF EXECUTIVE OFFICER Steve Choate CHIEF OPERATING OFFICER Bilal Malik, Ph.D. CHIEF SCIENCE OFFICER Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.
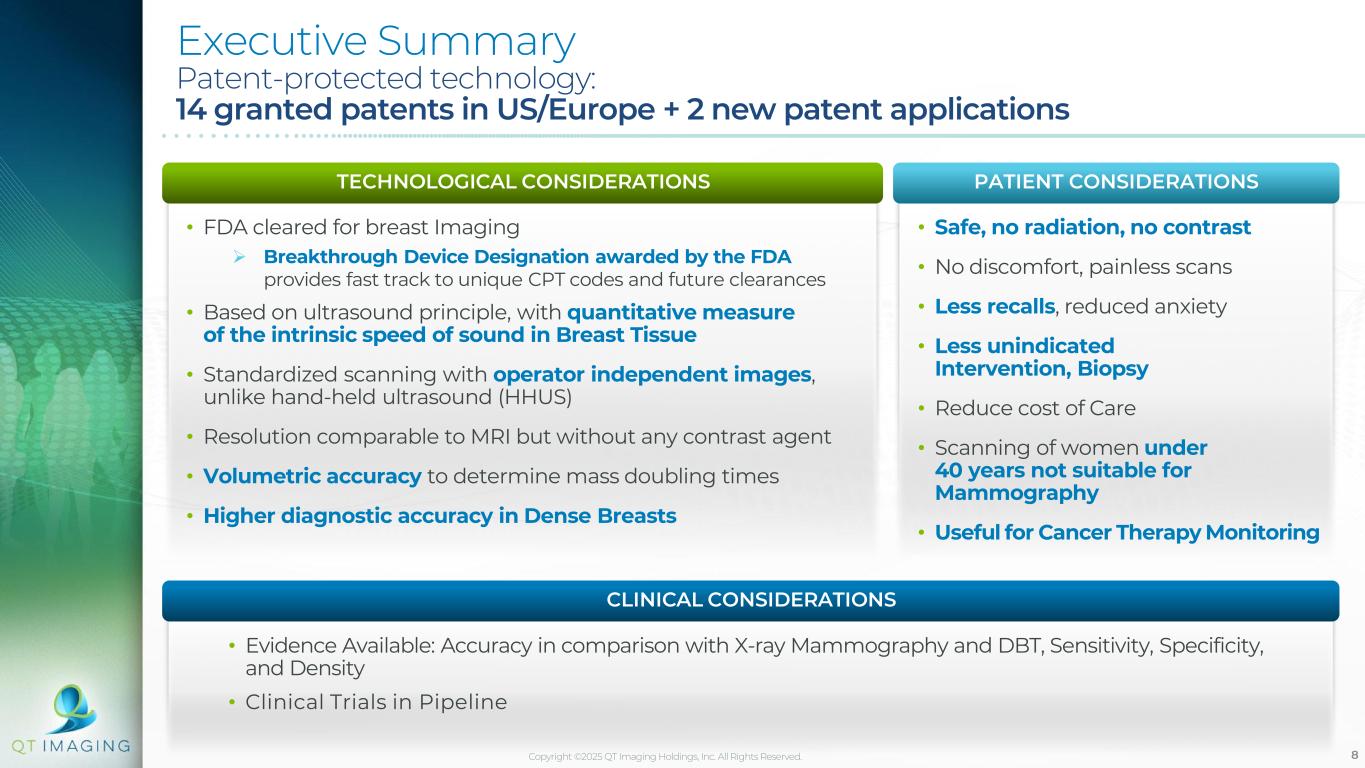
8 • FDA cleared for breast Imaging ➢ Breakthrough Device Designation awarded by the FDA provides fast track to unique CPT codes and future clearances • Based on ultrasound principle, with quantitative measure of the intrinsic speed of sound in Breast Tissue • Standardized scanning with operator independent images, unlike hand-held ultrasound (HHUS) • Resolution comparable to MRI but without any contrast agent • Volumetric accuracy to determine mass doubling times • Higher diagnostic accuracy in Dense Breasts • Safe, no radiation, no contrast • No discomfort, painless scans • Less recalls, reduced anxiety • Less unindicated Intervention, Biopsy • Reduce cost of Care • Scanning of women under 40 years not suitable for Mammography • Useful for Cancer Therapy Monitoring Executive Summary Patent-protected technology: 14 granted patents in US/Europe + 2 new patent applications TECHNOLOGICAL CONSIDERATIONS PATIENT CONSIDERATIONS • Evidence Available: Accuracy in comparison with X-ray Mammography and DBT, Sensitivity, Specificity, and Density • Clinical Trials in Pipeline CLINICAL CONSIDERATIONS Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

9 • Under Distribution Agreement with NXC Imaging (Subsidiary of Canon Medical Systems) for U.S.A. market − Committed quarterly minimum order quantities (MOQs) for scanners’ shipments till end of 2026 − Four additional distributors signed by NXC Imaging to cover sales across all states • Under Contract Manufacturing Agreement with Canon Medical Systems − In the process of bringing up large scale manufacturing with CMSC in Japan − QTI Novato site to continue manufacturing scanners Business Partnerships Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. 2025 Q1 • Q2 • Q3 • Q4 2026 Q1 • Q2 • Q3 • Q4 6 10 12 12 40 13 15 15 17 60

Breast Health

11 • New market opportunity given limitations of current imaging modalities for infants • Commenced feasibility study • Variety of image-guided procedures including biopsies, injections and cryoablation QTI’s Technology Has the Opportunity to Transform Several Large Markets CURRENT MARKET • FDA approved as supplementary screening device for breast imaging • Aim to revolutionize current imaging paradigm, replacing mammography, ultrasound (handheld and automated), and freeing MRI scanners time INFANT: $8B MARKET (4) IMAGE-GUIDED PROCEDURES: $5B MARKET (5)BREAST: $5B MARKET (2) ORTHO: $9B MARKET (3) • Target replacing MRI examinations • Primary focus on orthopedic practices (1) Medical Imaging Market Size, Share & Trends Analysis Report by Products (X-Ray, Ultrasound, Computed Tomography, Magnetic Resonance Imaging (MRI), Nuclear Imaging), by End Users (Hospitals, Diagnostic Imaging Centers, Other End Users), by Region (North America, Europe, Asia Pacific, Latin America, Middle East & Africa) - Global Industry Assessment (2016 - 2021) & Forecast (2023 - 2030), Vantage Market Research (2) Coherent Market Insights (3) Global Orthopedic Medical Imaging Systems Market Analysis Report 2022: Market to Reach $10.6 Billion by 2026 - The US Corners Orthopedic Medical Imaging Market with Adoption of Innovative Systems, Research and Markets. (4) Pediatric Imaging Market Size, Share & Trends Analysis Report By Modality (X-ray, Ultrasound, MRI, CT), By Application (Gastroenterology, Cardiology, Oncology), By End User, By Region, And Segment Forecasts, 2020 – 2027, Grandview Research. (5) Image-guided Therapy Systems Market Size, Share & Trends Analysis Report By Product (Ultrasound Systems, Computed Tomography Scanners), By Application, By End-use, And Segment Forecasts, 2022 – 2030, Grandview Research. 2023 Global Medical Imaging Market Size: $40B(1) FUTURE MARKETS – BODY SCANNER PLATFORM DEVELOPMENT Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

12 Many Women Have Dense Breasts, Which Mammograms are Inefficient in Screening for Cancer The FDA Has Recognized the Importance of Breast Density in Breast Cancer Screening QT Imaging’s FDA-cleared Solution for Dense Breasts 50% of women between the ages of 40-74 in the US have dense breasts(1) 50%50% Mammography Misses 35.6–52.2% of Breast Cancers in Dense Breast Tissue(4) (1) Breast Density on a Mammogram, Susan G. Komen (2) QTI Study | Dense Breast Mass Detection (3) “Mammograms Must Include Breast Density Information, New FDA Rule Says”. Wall Street Journal (4) The Role of Ultrasound in Screening Dense Breasts. NCBI. X-Ray Mammogram QT Scan (3) “the new rule advises physicians and patients to consider breast density alongside other cancer risk factors when deciding whether additional screening is necessary” – Hilary Marston, CHIEF MEDICAL OFFICER, FDA In ~84% of cases observed in a recent mini-study, QT Scanner identified abnormalities in dense breasts that were not identified by x-ray mammograms(2) Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

13 Clinical Evidence: Non-Inferiority to DBT The line closer to 1.0 is indicative of higher accuracy • Breast Abnormalities • Benign, non-cancer, normal without biopsy • Cancer, abnormal with biopsy • Different types of breast lesions (solid, cysts, complex) The line closer to 1.0 is indicative of higher accuracy Ref : A MultiReader Multicase (MRMC) Receiver Operating Characteristic (ROC) Study Evaluating Noninferiority of Quantitative Transmission (QT) Ultrasound to Digital Breast Tomosynthesis (DBT) on Detection and Recall of Breast Lesions : Yulei Jiang, PhD, Elaine Iuanow, MD, Bilal Malik, PhD,, John Klock, MD, Academic Radiology, Vol 31, No 6, June 2024. Non-inferiority to DBT ROC curves Individual reader AUC Comparison of AUC for 24 readers Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. QTI technology is a potential alternative to mammography for breast cancer screening of women too young to undergo DBT.

14 Sensitivity and Specificity • Sensitivity − Lower for QT (70.6%) compared to DBT (85.2%) − Potentially attributable to reader unfamiliarity with QT imaging, suggesting a need for enhanced training • Specificity − Significantly higher for QT (60.1%) compared to DBT (37.2%) − Indicates QT's ability to better differentiate benign from malignant lesions Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. Modality Average ± SD (%) 95% CI* Sensitivity DBT 85.2 ± 6.4 [83.1, 87.1] QT 70.6 ± 7.2 [68.3, 72.8 QT-DBT -14.6 ± 8.9 [17.2, -11.7] Specificity DBT 37.2 ± 11.0 [33.6, 40.7] QT 60.1 ± 12.3 [56.4, 64.0] QT-DBT 22.9 ± 10.5 [19.8, 26.1] Sensitivity and Specificity Based on Call-back vs. No Call-back Decisions of 24 Readers and 177 Cases (66 Abnormal, 111 Normal) CI, confidence interval; DB, digital breast tomosynthesis; QT, quantitative transmission; SD, standard deviation. *Estimated from 1,000 bootstrapping samples of the source data.

15 • Both sensitivity and specificity of DBT are dependent on breast density • Specificity of QT is independent of breast density Subgroup Analysis: Dense Breasts Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. BI-RADS Density N AUC ± SE 95% CI Abnormal/Normal QT DBT QT-DBT c, d 28/53 0.6852 ± 0.0457 0.5987 ± 0.0447 0.0865 ± 0.0557 [-0.0227, 0.1956] a, b 38/58 0.7912 ± 0.0335 0.7791 ± 0.0325 0.0121 ± 0.0242 [-0.0353, 0.0596] MRMC Analysis Results by Breast Density of 24 Readers and 177 Cases (66 Abnormal, 111 Normal)

16 Clinical Evidence Anatomic & Visual Grade with Comparative Modality QTI technology is highly accurate in visualizing the ductal and glandular tissue, even in dense breasts where such visualization can be challenging using conventional breast imaging technologies like XRM and/or HHUS. • 4 readers • 22 breast, 20 subjects • Lower score means better visualization • Visual Graded Analysis • Compared QTI vs HHUS, XRM • Graded Equivalent or Better than XRM/HHUS • 5 readers • 17 breast, 17 subjects • Lower score means better visualization ** ACR: American College Of Radiology Ref : John C Klock, Elaine Iuanow, Kathleen Smith, Nancy A and Obuchowski (2017) Visual Grading Assessment of Quantitative Transmission Ultrasound Compared to Digital X-ray Mammography and Hand-held Ultrasound in Identifying Ten Breast Anatomical Structures. Clinical Trials 3: 015 Normal Anatomic Comparison X-Ray Mammography (XRM) Handheld Ultrasound (HHUS) Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

17 35% 65% Do not follow guidelines Follow guidelines Over 80% of Callback Biopsies are Benign(4) 98% of Recalls are Avoidable (1) Mammography. Center for Disease Control and Prevention (2)Very Well Health | 13 Reasons for a Mammogram Callback | Larell Scardelli (3)PubMed | False-Negative Rate of Combined Mammography and Ultrasound for Women with Palpable Breast Masses | Carlos H.F. Chan, Suzanne B. Coopey, Phoebe E. Freer, and Kevin S. Hughes (4)National Breast Cancer Foundation | Breast Biopsy: Procedure Types, What to Expect and Results (5)U.S. Breast Cancer Statistics. Breastcancer.org. The Current Breast Imaging Paradigm Leads to Unnecessary Concern and Costs CALL BACK RATES ~15% call-backs rates with mammography BIOPSIES ~10% biopsy rate for callbacks CANCER INCIDENCE 0.3% cancer diagnosis(5) 150 15 3 For every 1,000 screening mammograms: 25% women do not get regularly screened and 35% of women with greater health related social needs (1) Screening compliance is low Of the 65% of women who do get screened, many suffer through unnecessary callbacks Aside from the discomfort of the mammogram procedure, up to 15% of women are called back for additional procedures such as ultrasound, MRI or biopsies – which can be expensive, time consuming and cause significant anxiety(2) Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

18 • Recall Rates: 10% Combined Recall Rate • Adherence to screening compliance: − 16% Decrease in Non-Cancer recall − 2% Decrease in Cancer Recall Clinical Evidence Recall Rate Approximately 150 women out of every 1,000 screened are recalled for more tests. Out of those 150, only three or four will be diagnosed with cancer. Unnecessary recalls create stress for the patient and have other negative impacts on and the breast healthcare industry. • Recall Rate is a metric to assess accuracy and detection rate • Anxiety Reducing Factor QTI technology improves non-cancer recall rates without substantially affecting cancer recall rates *An Exploratory Multi-reader, Multi-case Study Comparing Transmission Ultrasound to Mammography on Recall Rates and Detection Rates for Breast Cancer Lesions Bilal Malik, PhD, Elaine Iuanow, MD, John Klock, MD, Academic Radiology, Vol 29, No S1, January 2022 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Competitive Landscape Standard of Care Today and How QTI Fits In

20 Standard of Care Today 1, 2 How QT Scan Fits In (1) J Am Coll Radiol. 2023 Sep;20(9):902-914. (2) J Am Coll Radiol. 2024 Jun;21(6S):S126-S143 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. Patient age and risk assessment determines the screening pathway Patient over the age of 40 undergoes routine mammographic screening Patient under the age of 40 undergoes risk assessment Breast MRI recommended by guidelines Patient is above- average risk Patient is high-risk Patient is average or intermediate risk Standard mammography/ DBT screening Patient and Physician decide on the imaging modality Patient undergoes breast MRI Patient undergoes QT Scan as an alternative MRI findings evaluated QT Scan findings evaluated Results feed into diagnostic workup and follow-up plan Potential change to current clinical paradigm

21 Risk Category Lifetime Risk Breast Density Recommended Imaging Modalities Guideline Recommendations Average Risk ≤12–15% Fatty Breasts Screening Mammography (2D or 3D) annually starting at age 40 NCCN(4): Annual mammography for women aged 40 and older. ACR/SBI(1,2): Annual mammography starting at age 40. EUSOBI(3): Biennial mammography for women aged 50–69; consider starting at 40. Average Risk ≤12–15% Dense Breasts Screening Mammography (2D or 3D) annually starting at age 40 Supplemental Imaging: Consider Ultrasound or MRI NCCN: Consider supplemental imaging for women with heterogeneously or extremely dense breasts. ACR/SBI: Recommend supplemental MRI for women with dense breasts and additional risk factors. EUSOBI: Recommend MRI screening every 2–4 years for women aged 50–70 with extremely dense breasts. Above Average Risk 15–19% Any Density Screening Mammography (2D or 3D) annually starting at age 40 Supplemental Imaging: Consider MRI or Ultrasound NCCN: Annual mammography; consider MRI for women with a 20–25% lifetime risk. ACR/SBI: Recommend MRI for women with a 20–25% lifetime risk. EUSOBI: MRI screening for women with a 15–20% lifetime risk. High Risk ≥20–25% Any Density Screening Mammography (2D or 3D) annually starting at age 30 Supplemental Imaging: Annual MRI starting at age 25–30 NCCN: Annual MRI and mammography for women with ≥20% lifetime risk. ACR/SBI: Recommend annual MRI and mammography for women with ≥20% lifetime risk. EUSOBI: Recommend annual MRI for women with BRCA mutations or equivalent risk. Current Standard of Care in Breast Imaging (1) J Am Coll Radiol. 2023 Sep;20(9):902-914. (2) J Am Coll Radiol. 2024 Jun;21(6S):S126-S143 (3) Eur Radiol. 2024 Oct;34(10):6348-6357 (4) J Natl Compr Canc Netw. 2023 Sep;21(9):900-909 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

22 QTI’s Current Indications For Use Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. Broad intended use to allow breast imaging of any subject of age 18 or older First FDA clearance for an ultrasound-based device to be able to quantify breast tissue volume QT Scanner 2000 Model A 510(k) Summary (link)

23 How QTI Potentially Fits Into the Current Paradigm Risk Category Potential Role of QTI Device Average Risk (≤12–15%) QTI offers a non-ionizing, high-resolution alternative for supplemental imaging, especially useful in patients with dense breasts where mammography is limited. Ideal for frequent monitoring without radiation exposure. Above-Average Risk (15–19%) QTI provides a safer alternative to MRI for moderate-risk individuals, including those with family history or dense tissue. It avoids gadolinium-based contrast risks, offering functional imaging with fewer contraindications. High Risk (≥20–25%) QTI may supplement or replace MRI in high-risk individuals, especially where MRI is contraindicated or poorly tolerated. Supports early, radiation-free surveillance with improved soft-tissue contrast, aligning with early screening needs. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

QTI Technology vs HHUS, DBT, MRI, CT

25 Imaging Modalities Breast Imaging Modality Acronym Underlying Technology QT Scan QT Scan Ultrasound Mammography XRM X-Ray Digital Breast Tomosynthesis DBT X-Ray Magnetic Resonance Imaging MRI Magnetic Resonance Contrast Enhanced Magnetic Resonance Imaging CE-MRI Magnetic Resonance + Contrast Breast Computed Tomography Breast CT X-Ray Handheld Ultrasound HHUS Ultrasound Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

26 The QT Scanner Delivers a Better Experience for Patients than Traditional Systems QT Scan CE-MRIHHUS XRM/DBT The QTI Imaging Advantage ...OVER XRM/DBT • Improved image quality • Safer (no radiation), allowing for more frequent imaging • Greater specificity • No special facility requirements • Quantifiable/repeatable …OVER HHUS • Superior image quality • Not operator dependent • Quantifiable/repeatable …OVER MRI • High resolution and contrast-to-noise ratio • No injection needed • Lower equipment cost • No special facility or shielding requirements (1) No radiation exposure or injections necessary Breast CT ...OVER BREAST CT • No radiation – breast CT radiation is significantly higher than screening mammography • No contrast needed (compared to contrast enhanced CT) 40-45 min 10-30 min 40-45 min 5-10 minutes 5 minutes https://www.bcrf.org/about-breast-cancer/breast-ultrasound/ https://pmc.ncbi.nlm.nih.gov/articles/PMC10183872/?utm_source=chatgpt.com https://winshipcancer.emory.edu/cancer-types-and-treatments/breast- cancer/screening.php?utm_source=chatgpt.com https://www.koninghealth.com/about-koning/frequently-asked-questions Ultrasound Ultrasound Magnetic Resonance X-Ray X-Ray Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. Underlying Technology Image Quality Safety(1) Speed Cost Efficiency Patient Experience

27 INVENIA ABUS ACUSON S2000 ABVS AWBUS SOFIA 3D DELPHINUS SOFTVUE BREAST ACOUSTIC CT DESIGN TYPE Articulating Arm Articulating Arm Articulating Arm Guided Handheld Rotating Armature Water Bath Water Bath OUTPUT Stacked 2D Reflection Slices Stacked 2D Reflection Slices Stacked 2D Reflection Slices Stacked 2D Reflection Slices Stacked 2D Transmission & Reflection Slices Only Full 3D – transmission & reflection volumes Other Ultrasound Products Use 2D Imaging for Dense Breast Imaging QTI enhances specificity by taking advantage of the speed of sound information, which Is unavailable (or lower quality) with the competing technologies Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

28 Current Ultrasound Technologies Have Major Deficiencies Shortfalls of Commercial Current, Rival Systems (2) • Reflection images suffer from speckle; compounding is done without refraction correction • No true “transmission” mode available – instead use low resolution “shear wave” data (e.g. ABUS, AVUS are not transmission systems) • Data is compounded 2D, not true 3D - transmission images often contain artifacts • Low contrast-to-noise ratio due to speckle • Specificity for identifying masses is relatively poor • Inconsistent visualization of calcifications – resulting in up to 12% of cancers being missed (1) • Conventional ultrasound lacks consistent specific tissue volume segmentation and not FDA cleared for quantitative breast density estimates • Poor reproducibility of measurements and volume data (3) • High operator dependency in lesion characterization (4) 28 (1) Skaane P, Sauer T. Ultrasonography of malignant breast neoplasms. Analysis of carcinomas missed as tumor. Acta Radiol. 1999;40:376–82 (2) Based on opinion of QTI management. QTI believes necessary data has been obtained through 18 separate clinical trials (3) Diagnostics (Basel). 2024 Jul 25;14(15):1602 (4) Radiology. 2006 Nov;241(2):355-65 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

29 QTI Technology vs XRM/DBT 29 • Projection overlap in mammography: overlapping tissues in 2D mammograms can obscure or mimic lesions(3) (DBT improves this, but not completely eliminates it) • Reduced sensitivity to dense breasts: Mammography and DBT can miss cancers in women with dense breast tissue(4) • Radiation exposure: Although low, there is still ionizing radiation exposure, especially with DBT, which may slightly increase cumulative lifetime risk of cancer. Diagnostic mammograms result in even higher radiation exposure(1) • Limited detection of certain cancers: Some types of cancers, such as invasive lobular carcinoma, are harder to detect with mammography/DBT(5) • Overdiagnosis: Detection of slow-growing cancers that might no impact a patient’s lifespan, leading to overtreatment(6) • Compression discomfort: Breast compression during imaging is uncomfortable and can deter regular screening • Limited visualization in patients with implants: Breast implants can obscure underlying tissue in mammography/DBT, making it more difficult to detect tumors • Breast density: Lack of volumetric imaging results in incorrect quantitative estimate of breast density as well as reader disagreement(2,3) (1) Eur Radiol. 2025 Jan;35(1):166-176 (2) AJR Am J Roentgenol 2013 Sep;201(3):692-7. doi: 10.2214/AJR.12.10197. (3) Med Phy. 2015 Dec;42(12):7059-7 (4) Eur Radiol. 2017 Jul;27(7):2744-2751 (5) Eur J Surg Oncol. 2008 Feb;34(2):135-42 (6) N Engl J Med. 2016 Oct 13;375(15):1438-1447 Screening Mammogram Diagnostic Mammogram Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

30 QTI Technology vs MRI: Shortfalls of Breast MRI • High cost and limited availability: Breast MRI is expensive and not widely accessible compared other imaging modalities • Limited specificity: While highly sensitive, breast MRI often produces false positives, leading to unnecessary biopsies(1) • Contrast agent dependency: Most breast MRIs rely on gadolinium- based contrast agents, which carry risks, especially for patients with kidney issues and gadolinium retention concerns(5) • Patient comfort: MRI exams can be uncomfortable due to awkward prone positioning, noise, and confinement in the scanner(3) • Variable image quality: Image quality can vary based on patient movement, breast size, or technical factors like coil design and magnet strength • Lack of standardization: Differences in imaging protocols across institutions can complicate interpretation and comparison of results(2) • Technical complexity: Requires specialized room and technicians trained in breast MRI protocols, limiting widespread use(4) • Breast density: No FDA-cleared method or algorithm available for breast density assessment 30 Non-fat sat Pre-contrast Post-contrastFL3D (1) Br J Radiol. 2012 Mar;85(1011):197–207 (2) Curr Probl Diagn Radiol. 2020 Sep-Oct;49(5):312-316 (3) Top Magn Reson Imaging. 2020 Jun;29(3):125-130 (4) J Magn Reson Imaging. 2015 Sep;42(3):566-71 (5) "FDA drug safety communication" US Food and Drug Administration (2018). Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

31 Quantitative Transmission (QT) Imaging • What is QT Imaging? − Inherently 3D volumetric ultrasound modality due to 3D data acquisition and image reconstruction − Uses CT-like configuration with ultrasound to acquire and reconstruct transmission images which map the speed-of-sound across the tissue volume − High resolution, similar to MRI − Images tissue without overlap, providing more information than conventional HHUS − Overcomes operator dependence and lack of standardization associated with HHUS − Pain free, safe • Image Acquisition: − Prone position with breast submerged in water − 360-degree rotation of ultrasound arrays − 10-12 minutes per breast average scan time Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved. Reflection transducers Transmission mode receiver Transmission mode transmitter

32 Optimized Patient Experience • No ionized radiation. Acoustic source only • No breast compression and associated discomfort • 10-12 minutes per breast exam time • Quiet and comfortable (as compared to MRI - claustrophobia, coil pressure, noise and lengthy exams) • No contrast injection or associated risk (as compared to MRI Gadolinium) • No limitations for dense breasts or implants PerfeQTion Imaging Center Haverford, PA Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Clinical Results Comparison

34 Normal Breast Dense Breast Cyst Tumor Solid Tumor Calcification Quantitative Tissue / Density Characterization Implant Visualization Imaging Accuracy in Breast Mass Diagnosis(1) QT Scan HHUSXRM/DBT CE-MRI (1) Based on opinion of QT Imaging Holdings team. (2) Quantitaitve tissue/density characterizxation means assessment of quantitative/volumetric breast density. Other than Mammography and QTI, there are no FDA cleared algorithms for volumetric density assessment CT Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Technology & Clinical Overview

36 Technical Capabilities • Detection resolution of ~600 microns in reflection compared to 800 microns(1) for MRI (depends on field strength, homogeneity etc) • Contrast to noise ratio of 23:1 at 100 microns ( in reflection; can detect small calcifications) • Contrast to noise ratio of 15:1 (at resolution in transmission – speed of sound) • Speckle-free because of 3600 compounding and refraction correction for reflection image • Volumetric data acquisition (3D), not stacked 2D slices • Volumetric reproducibility 0.2% for fibro glandular volume • Volumetric accuracy better than 3% extrapolated from linear accuracy ~1% ( vertical < 2%) (1) Y. Gao and S. L. Heller, "Abbreviated and Ultrafast Breast MRI in Clinical Practice," RadioGraphics, vol. 40, pp. 1507-1527, 2020 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

37 QTI Provides High Resolution, Similar to MRI MRI QTI (3D UT) VolumetricPlanar QT redundancy of data means: • Similar collection time and resolution • Higher detection capability • Higher Signal-to-Noise (without Gadolinium or other contrast) • Repeatable quantitative measurements • Quantitative and morphological biomarkers for longitudinal studies Data points per voxel ~36 billion data points 22 million voxels Data points per voxel ~180 thousand data points (1) 20mm overlapping detectors Receiver Array Receiver Array 2mm gap Spiral data acquisition for speed of acquisition Note: Voxel is a 3D version of a pixel ~200,000 times more data per voxel than (N MRI ) (1) Y. Gao and S. L. Heller, "Abbreviated and Ultrafast Breast MRI in Clinical Practice," RadioGraphics, vol. 40, pp. 1507-1527, 2020 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

38 Enhanced Clinical Capabilities and Value (1) Y. Gao and S. L. Heller, "Abbreviated and Ultrafast Breast MRI in Clinical Practice," RadioGraphics, vol. 40, pp. 1507-1527, 2020 • High-quality and high-resolution native 3D Imaging • Quantifiable images enables accurate analysis, comparison and trending • Consistent and reproduceable image quality regardless of operator or breast size/tissue type • Clinical feature detection of 50-100 microns including microcalcifications • Functional imaging capability - determine tissue type from the speed of sound • Allows tissue doubling time assessments – similar to MRI and CT • Highly accurate measurements, not scanner operator dependent Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

39 QT Speed of Sound and Reflection Images Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

40 Modality Comparison – FFDM and HHUS Mammogram - (left) CC and (right) MLO views. Arrows mark a region of spiculated focal asymmetry. HHUS images across the lesion Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

41 Modality Comparison – MRI Images Non-fat sat Pre-contrast Post-contrastFast low angle shot 3D (FL3D) Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

42 Modality Comparison – QT Image QT speed of sound image showing the mass (marked by arrows) as a region of high-speed IDC in lower outer quadrant of the left breast, 4 o’clock in the coronal view. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

QTI Scans Image Quality

44 Normal Dense Breast CORONAL AXIAL SAGITTAL Transmission Reflection Dense breast – no high-speed lesion, mass, or cancer click on image for video Chest wall Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

45 Cyst Identification Using Speed of Sound Cystic mass seen in speed of sound (top 3 panels) and anechoic in reflection (bottom 3 panels) in 3 planes – coronal, axial, and sagittal Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

46 Solid Identification Using Speed of Sound Solid mass seen in speed of sound (top 3 panels) and hypoecoic in reflection (bottom 3 panels) in 3 planes – coronal, axial and sagittal Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

47 Case 1: Invasive Ductal Carcinoma Mammogram - (left) CC and (right) MLO views Arrows mark a region of spiculated focal asymmetry HHUS images across the lesion Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

48 Case 1: MRI Non-fat sat Pre-contrast Post-contrastFL3D Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

49 Case 1: QTI Scan QT speed of sound image showing the mass (marked by arrows) as a region of high-speed IDC in lower outer quadrant of the left breast, 4 o’clock in the coronal view. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

50 Case 2: MRI – Invasive Ductal Carcinoma Non-fat sat Pre-contrast Post-contrastFL3D Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

51 Case 2: QTI Speed of Sound QT speed of sound image showing the mass (marked by arrows) as a region of high-speed. In coronal view at 8 o’clock. Relatively posterior mass but still all visible in the QT image. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

52 Case 3: MRI – Invasive Ductal Carcinoma Non-fat sat Pre-contrast Post-contrastFL3D Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

53 Case 3: QTI Speed of Sound QT speed of sound image showing the mass (marked by arrows) as a region of high-speed. In coronal view, at 6 o’clock, near the center. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

54 Calcifications Detected with Better Visibility Than XRM (1) Sensitivity of Quantitative Transmission ultrasound to detection of microcalcifications. SPIE (International Society for Optics and Photonics) Meeting Houston Texas February 20, 2018. Digital Mammography QT Reflection Tomogram Detectability of calcifications in QT Acoustic CT is superior to XRM (1) Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Market Positioning & QT Scanner Locations In USA

56 Not intended to compete with mammography for screening, although many patients may find it preferrable for: • Dense breasts • Implants • Post therapy screening where breasts can be sensitive to compression • When concerned about radiation dose Diagnostic alternative to MRI • Lower cost, faster, more accessible • Similar image quality and diagnostic value • More tolerable for patient (claustrophobia, noise, time, no contrast) • Images are inherently quantitative and repeatable, and hence serve as an imaging biomarker (helps following a patient) • Scanner is easily deployable (<2 days) and frees MRI scanners for other non-breast imaging studies Diagnostic alternative to Hand-held Ultrasound • Native 3D imaging (like MRI and CT) • Quantifiable image analysis • No need for specialized technologist training • Consistent and reproducible image quality regardless of operator Market Positioning of Breast Acoustic CT Scanner Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

57 Center For New Medicine Dr. Leigh Erin Connealy 6 Hughes, Suite 100 Irvine, CA 92618 +1 (949) 680-1880 Website Couri Center for Gynecology and Integrative Women's Health Dr. Michele Couri 6708 N Knoxville Ave, Suite 1 Peoria, IL 61614 Website Innovative Radiology Dr. John Tentinger 7601 Office Plaza Dr, Ste 115 West Des Moines, IA 50266 +1 (515) 222-0550 Website PerfeQtion Imaging Dr. Jenn Simmons 346 W Lancaster Ave, Haverford PA 19041 Website PerfeQtion Imaging Novato Dr. John Klock 3 Hamilton Landing #180 Novato, CA 94949 +1 (415) 842-7403 Email Qlarity Breast Imaging Dr. Kristine Burke True Health Center for Precision Medicine 8105 Saratoga Way, #240 El Dorado Hills, CA 95762 +1 (916) 542-1644 Website Qlarity Breast Imaging Dr. Yvonne Karney Vitality Renewal Functional Medicine 31 N. Virginia St. Crystal Lake, IL 60014 +1 (815) 271-7300 Website Vincere Cancer Center Dr. Vershalee Shukla and Dr. Pablo Prichard Top Cancer Center in Scottsdale, AZ Vincere Cancer Center 7469 E Monte Cristo Ave. Scottsdale AZ 85260 +1 (480) 306-5390 Website Keio University 2 Chome-15-45 Mita Minato City, Tokyo 108-007 Japan National Institutes of Health (NIH) 9000 Rockville Pike Bethesda, MD 20892 United States Sunnybrook Health Sciences Center (NIH Grant) 2075 Bayview Ave North York, ON M4N 3M5 Canada QT Scanner Locations Map COMMERCIAL CENTERS CLINICAL SITES Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Open Angle Scanner

59 Developing an Open Angle Scanner Will Expand the Technology to New Markets …providing significant potential to access new markets and applications • The Open Angle Scanner uses an open, partial angle configuration which reduces the viewing field from 3600 to 3250 and provides additional capabilities for QTI technology in: − Orthopedic imaging − Prostate imaging − Whole body infant scanning − Biopsy and image-guided diagnostic and treatment procedures • The scanner satisfies the need for better image reconstruction techniques in partial-ring tomography systems • Potential to prevent cancers from developing into advanced stages • Representative point-of-care target markets include: ORTHOPEDIC SURGEONS [IN-OFFICE] SPORTS TEAMS [ON THE FIELD] MILTARY [SHIPS & FIELD USE] Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Financials

61 Financial Highlights for Q1’25 • Shipped six scanners and generated revenue of $2.8 Million with 65% gross margin in the first quarter of 2025 • Closed $10.1 million Lynrock Lake term loan to: − Retire prior debt and − Provide $5.4 million for working capital purposes • Entered into Contract Manufacturing Agreement with Canon Medical Systems Corporation for large scale manufacturing − QTI Novato will continue manufacturing in parallel until full transfer • Announced PIPE investment of $0.7 Million, funded by QTI Board of Directors Members and other investors Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

62 Financial Highlights for Q1’25 QTD • Commercial revenue was $2.8 million during the first quarter of 2025, compared to $0.8 million during the fourth quarter of 2024 and $1.4 million during the first quarter of 2024 • Gross margin of 65% in the first quarter of 2025, compared to gross margin of 47% in the fourth quarter of 2024 and 56% in the first quarter of 2024 − The increase in margin in the first quarter of 2025 compared to the fourth quarter of 2024 and first quarter of 2024 was attributable to variability in the weighted average cost related to the Company's existing inventory • Net loss of $11.1 million for the first quarter of 2025, compared to net loss of $0.6 million for the first quarter in 2024 and net loss of $3.5 million for the fourth quarter in 2024. Q1’25 net loss included: − $0.7 million of net non-cash expense related to the change in fair value of warrants, derivative, and earnout liabilities − $2.1 million of modification and debt extinguishment expenses − $6.6 million of debt modification and extinguishment expense related to the issuance of the Lynrock Lake Term Loan, and − $0.1 million of stock-based compensation expense − compared to a net loss of $0.6 million for the first quarter of 2024, which included: ▪ $5.6 million of net non-cash income related the change in fair value of warrants, derivative, and earnout liabilities, and ▪ $4.3 million of one-time transaction expenses. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

63 Financial Highlights for Q1’25 QTD • Non-GAAP Adjusted EBITDA of $(0.9) million for the first quarter of 2025, compared to $(1.2) million for the first quarter in 2024 and $(1.9) million for the fourth quarter of 2024. • Ended Q1’25 with $3.0M in cash, compared to end of Q4’24 with $1.2M in cash, which includes $3.5 million used for operating activities, offset by net $5.4 million of financing activities related to proceeds received from the Lynrock Lake Term Loan and repayment of Yorkville and Cable Car Notes in Q1’25. • Reiterated plans to deliver $18 million in revenue in 2025 (shipment of 40 scanners) and $27 million in revenue in 2026 (shipment of 60 scanners). These targets are in accordance with the MOQs per our Amended Distribution Agreement with our strategic business and distribution partner, NXC Imaging, Inc., a wholly owned subsidiary of Canon Medical Systems USA. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

64 Summary of Q1’25 QTD GAAP Results Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

65 Summary of Q1’25 QTD Non-GAAP Results Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

66 Adjustments to EBITDA (1) The Company recorded debt modification expense of $0.1 million related to its modification of the Cable Car Note on January 9, 2025 and debt extinguishment expense of $2.0 million related to the extinguishment of the Yorkville Note and Cable Car Note on February 26, 2025 in other expense, net for the three months ended March 31, 2025. (2) The increase in fair value of warrant liability during the three months ended March 31, 2025 relates to the liability classified private placement warrants, the Lynrock Lake Warrant, and Yorkville Warrant, which is primarily driven by increase in the Company's stock price from the date of issuance of the Lynrock Lake Warrant and Yorkville Warrant and as of March 31, 2025. (3) The decrease in fair value of derivative liability during the three months ended March 31, 2025 related to the Yorkville Pre-paid Advance, which contained features that were bifurcated as freestanding financial instruments and initially valued on March 4, 2024 upon consummation of the Merger. The derivative liability was subsequently revalued as of February 26, 2025, prior to the extinguishment of the Yorkville Note. (4) The earnout liability relates to the contingent consideration for the Merger Earnout Consideration Shares pursuant to the Business Combination Agreement dated December 8, 2022, as amended in September 2023. The earnout liability was initially valued using the Monte Carlo Simulation method on March 4, 2024 and subsequently revalued using the same method as of March 31, 2025. (5) The Company incurred transaction expenses related to the Merger with GigCapital5, Inc., which closed on March 4, 2024. These transaction expenses included a $3.7 million of transaction costs that were settled with issuance of common stock, $0.4 million of transaction costs settled or payable in cash and a $0.2 million loss on issuance of common stock in connection with a subscription agreement, which were recorded as selling, general and administrative expenses in the condensed consolidated statement of operations during the three months ended March 31, 2024. There were no transaction expenses incurred during the three months ended March 31, 2025. (6) Upon the issuance of Lynrock Lake Term Loan closed on February 26, 2025, the Company recorded a loss of $6.6 million, including debt issuance costs of $0.2 million, in other expense, net for the three months ended March 31, 2025. Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

67 Balance Sheets as of Q1’25 and Q4’24 Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

68 Cash Flow Statements for Q1’25 YTD and Q1’24 YTD Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

69 Investment Highlights Cutting-edge imaging technology with multiple potential applications creates a tremendous opportunity to transform the imaging market Committed & Experienced Executive Suite Industry-Transforming, FDA Cleared (Breakthrough Device Designation) Imaging Technology Platform Recognized by Industry Incumbents Recent Changes to FDA Rules and USPSTF Guidance on Breast Screening Provide Meaningful Tailwinds and Momentum Differentiated Solution in Large and Important, $5B(1) Breast Screening Market Potential to Significantly Expand TAM Through Adjacent Market Applications NXC Imaging Distribution Agreement to Drive Accelerated Commercial Roll-out Manufacturing Agreement with Canon Medical to Drive Large Scale Manufacturing (1) Coherent Market Insights Copyright ©2025 QT Imaging Holdings, Inc. All Rights Reserved.

Thank You!